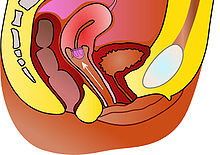
The cervix or cervix uteri is a dynamic fibromuscular organ of the female reproductive system that connects the vagina with the uterine cavity. The human cervix has been documented anatomically since at least the time of Hippocrates, over 2,000 years ago[citation needed]. The cervix is approximately 4 cm long with a diameter of approximately 3 cm and tends to be described as a cylindrical shape, although the front and back walls of the cervix are contiguous. The size of the cervix changes throughout a women's life cycle. For example, during their fertile years of the reproductive cycle, females tend to have a larger cervix vis á vis postmenopausal females; likewise, females who have produced offspring have a larger sized cervix than females who have not produced offspring.

A copper intrauterine device (IUD), also known as an intrauterine coil or copper coil or non-hormonal IUD, is a type of intrauterine device which contains copper. It is used for birth control and emergency contraception within five days of unprotected sex. It is one of the most effective forms of birth control with a one-year failure rate around 0.7%. The device is placed in the uterus and lasts up to twelve years. It may be used by women of all ages regardless of whether or not they have had children. Following removal, fertility quickly returns.

Fertility awareness (FA) refers to a set of practices used to determine the fertile and infertile phases of a woman's menstrual cycle. Fertility awareness methods may be used to avoid pregnancy, to achieve pregnancy, or as a way to monitor gynecological health.

The diaphragm is a barrier method of birth control. It is moderately effective, with a one-year failure rate of around 12% with typical use. It is placed over the cervix with spermicide before sex and left in place for at least six hours after sex. Fitting by a healthcare provider is generally required.
Spermicide is a contraceptive substance that destroys sperm, inserted vaginally prior to intercourse to prevent pregnancy. As a contraceptive, spermicide may be used alone. However, the pregnancy rate experienced by couples using only spermicide is higher than that of couples using other methods. Usually, spermicides are combined with contraceptive barrier methods such as diaphragms, condoms, cervical caps, and sponges. Combined methods are believed to result in lower pregnancy rates than either method alone.

A menstrual cup is a menstrual hygiene device which is inserted into the vagina during menstruation. Its purpose is to collect menstrual fluid. Menstrual cups are made of elastomers. A properly-fitting menstrual cup seals against the vaginal walls, so tilting and inverting the body will not cause it to leak. It is impermeable and collects menstrual fluid, unlike tampons and menstrual pads, which absorb it.
A pessary is a prosthetic device inserted into the vagina for structural and pharmaceutical purposes. It is most commonly used to treat stress urinary incontinence to stop urinary leakage and to treat pelvic organ prolapse to maintain the location of organs in the pelvic region. It can also be used to administer medications locally in the vagina or as a method of contraception.

The contraceptive sponge combines barrier and spermicidal methods to prevent conception. Sponges work in two ways. First, the sponge is inserted into the vagina, so it can cover the cervix and prevent any sperm from entering the uterus. Secondly, the sponge contains spermicide.

A hormonal intrauterine device (IUD), also known as an intrauterine system (IUS) with progestogen and sold under the brand name Mirena among others, is an intrauterine device that releases a progestogenic hormonal agent such as levonorgestrel into the uterus. It is used for birth control, heavy menstrual periods, and to prevent excessive build of the lining of the uterus in those on estrogen replacement therapy. It is one of the most effective forms of birth control with a one-year failure rate around 0.2%. The device is placed in the uterus and lasts three to eight years. Fertility often returns quickly following removal.

Vaginal rings are polymeric drug delivery devices designed to provide controlled release of drugs for intravaginal administration over extended periods of time. The ring is inserted into the vagina and provides contraception protection. Vaginal rings come in one size that fits most women.
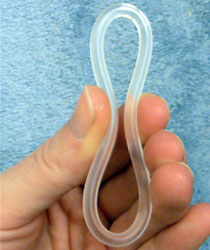
A contraceptive vaginal ring is a type of hormonal insert that is placed in the vagina for the purpose of birth control. The rings themselves utilize a plastic polymer matrix that is inlaid or embedded with contraceptive drug. This drug, often one or two hormones, is absorbed directly through the bloodstream through the cells that line the vaginal wall. Some vaginal rings contain both an estrogen and a progestin, which are available in Europe and the United States. Other vaginal rings contain just progesterone. The progesterone-only ring is only available in Latin America, exclusively for postpartum breastfeeding parents.
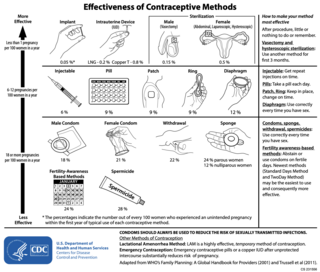
″

Birth control, also known as contraception, anticonception, and fertility control, is the use of methods or devices to prevent unintended pregnancy. Birth control has been used since ancient times, but effective and safe methods of birth control only became available in the 20th century. Planning, making available, and using human birth control is called family planning. Some cultures limit or discourage access to birth control because they consider it to be morally, religiously, or politically undesirable.
A conception device is a medical device which is used to assist in the achievement of a pregnancy, often, but not always, by means other than sexual intercourse. This article deals exclusively with conception devices for human reproduction.

An intrauterine device (IUD), also known as intrauterine contraceptive device or coil, is a small, often T-shaped birth control device that is inserted into the uterus to prevent pregnancy. IUDs are one form of long-acting reversible birth control (LARC). One study found that female family planning providers choose LARC methods more often (41.7%) than the general public (12.1%). Among birth control methods, IUDs, along with other contraceptive implants, result in the greatest satisfaction among users.

The womb veil was a 19th-century American form of barrier contraception consisting of an occlusive pessary, i.e. a device inserted into the vagina to block access of the sperm into the uterus. Made of rubber, it was a forerunner to the modern diaphragm and cervical cap. The name was first used by Edward Bliss Foote in 1863 for the device he designed and marketed. "Womb veil" became the most common 19th-century American term for similar devices, and continued to be used into the early 20th century. Womb veils were among a "range of contraceptive technology of questionable efficacy" available to American women of the 19th century, forms of which began to be advertised in the 1830s and 1840s. They could be bought widely through mail-order catalogues; when induced abortion was criminalized during the 1870s, reliance on birth control increased. Womb veils were touted as a discreet form of contraception, with one catalogue of erotic products from the 1860s promising that they could be "used by the female without danger of detection by the male."

CONRAD is a non-profit organization scientific research organization that works to improve the reproductive health of women, especially in developing countries. CONRAD was established in 1986 under a cooperative agreement between Eastern Virginia Medical School (EVMS) and the United States Agency for International Development(USAID). CONRAD’s products are developed primarily for women in low-resource settings, in that they are designed to be safe, affordable and user-friendly. CONRAD is led by Scientific and Executive Director Gustavo Doncel, M.D., Ph.D. Primary funding for CONRAD comes from the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Agency for International Development (USAID), with additional funding from The Bill & Melinda Gates Foundation and the National Institutes of Health (NIH).

Postcoital bleeding (PCB) is non-menstrual vaginal bleeding that occurs during or after sexual intercourse. Though some causes are with associated pain, it is typically painless and frequently associated with intermenstrual bleeding.

Combined hormonal contraception (CHC), or combined birth control, is a form of hormonal contraception which combines both an estrogen and a progestogen in varying formulations.

Cervical drug delivery is a route of carrying drugs into the body through the vagina and cervix. This is a form of localized drug delivery that prevents the drugs from impacting unintended areas of the body, which can lower side effects of toxic drugs such as chemotherapeutics. Cervical drug delivery has specific applications for a variety of female health issues: treatment of cervical cancer, pregnancy prevention, STD prevention, and STD treatment.