Related Research Articles

Emergency contraception (EC) is a birth control measure, used after sexual intercourse to prevent pregnancy.

The combined oral contraceptive pill (COCP), often referred to as the birth control pill or colloquially as "the pill", is a type of birth control that is designed to be taken orally by women. It is the oral form of combined hormonal contraception. The pill contains two important hormones: a progestin and estrogen. When taken correctly, it alters the menstrual cycle to eliminate ovulation and prevent pregnancy.

Levonorgestrel is a hormonal medication which is used in a number of birth control methods. It is combined with an estrogen to make combination birth control pills. As an emergency birth control, sold under the brand names Plan B One-Step and Julie, among others, it is useful within 72 hours of unprotected sex. The more time that has passed since sex, the less effective the medication becomes, and it does not work after pregnancy (implantation) has occurred. Levonorgestrel works by preventing ovulation or fertilization from occurring. It decreases the chances of pregnancy by 57–93%. In an intrauterine device (IUD), such as Mirena among others, it is effective for the long-term prevention of pregnancy. A levonorgestrel-releasing implant is also available in some countries.

A contraceptive patch, also known as "the patch", is a transdermal patch applied to the skin that releases synthetic oestrogen and progestogen hormones to prevent pregnancy. They have been shown to be as effective as the combined oral contraceptive pill with perfect use, and the patch may be more effective in typical use.
Progestogen-only pills (POPs), colloquially known as "mini pills", are a type of oral contraceptive that contain synthetic progestogens (progestins) and do not contain estrogens. They are primarily used for the prevention of undesired pregnancy, although additional medical uses also exist.

Drospirenone is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses. It is available both alone under the brand name Slynd and in combination with an estrogen under the brand name Yasmin among others. The medication is an analog of the drug spironolactone. Drospirenone is taken by mouth.
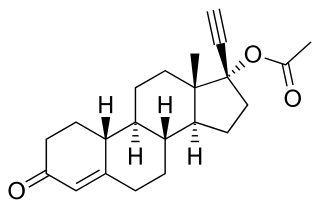
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is ingested orally.

Norgestimate, sold under the brand names Ortho Tri-Cyclen and Previfem among others, is a progestin medication which is used in birth control pills for women and in menopausal hormone therapy. The medication is available in combination with an estrogen and is not available alone. It is taken by mouth.

Norgestrel is a progestin which is used in birth control pills sold under the brand name Ovral in combination with the estrogen ethinylestradiol and Opill by itself. It is also used in menopausal hormone therapy. It is taken by mouth.

Vaginal rings are polymeric drug delivery devices designed to provide controlled release of drugs for intravaginal administration over extended periods of time. The ring is inserted into the vagina and provides contraception protection. Vaginal rings come in one size that fits most women.
Intermenstrual bleeding (IMB) is vaginal bleeding at irregular intervals between expected menstrual periods. It may be associated with bleeding with sexual intercourse.

Hormonal contraception refers to birth control methods that act on the endocrine system. Almost all methods are composed of steroid hormones, although in India one selective estrogen receptor modulator is marketed as a contraceptive. The original hormonal method—the combined oral contraceptive pill—was first marketed as a contraceptive in 1960. In the ensuing decades, many other delivery methods have been developed, although the oral and injectable methods are by far the most popular. Hormonal contraception is highly effective: when taken on the prescribed schedule, users of steroid hormone methods experience pregnancy rates of less than 1% per year. Perfect-use pregnancy rates for most hormonal contraceptives are usually around the 0.3% rate or less. Currently available methods can only be used by women; the development of a male hormonal contraceptive is an active research area.
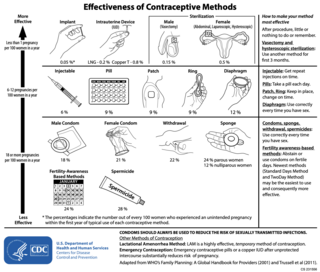
There are many methods of birth control that vary in requirements, side effects, and effectiveness. As the technology, education, and awareness about contraception has evolved, new contraception methods have been theorized and put in application. Although no method of birth control is ideal for every user, some methods remain more effective, affordable or intrusive than others. Outlined here are the different types of barrier methods, hormonal methods, various methods including spermicides, emergency contraceptives, and surgical methods and a comparison between them.

Dienogest, sold under the brand name Visanne among others, is a progestin medication which is used in birth control pills and in the treatment of endometriosis. It is also used in menopausal hormone therapy and to treat heavy periods. Dienogest is available both alone and in combination with estrogens. It is taken by mouth.
Birth control pills come in a variety of formulations. The main division is between combined oral contraceptive pills, containing both estrogens and synthetic progestogens (progestins), and progestogen only pills. Combined oral contraceptive pills also come in varying types, including varying doses of estrogen, and whether the dose of estrogen or progestogen changes from week to week.
Progestogen-only injectable contraceptives (POICs) are a form of hormonal contraception and progestogen-only contraception that are administered by injection and providing long-lasting birth control. As opposed to combined injectable contraceptives, they contain only a progestogen without an estrogen, and include two progestin preparations:

Ethinylestradiol/etonogestrel, sold under the brand names NuvaRing among others, is a hormonal vaginal ring used for birth control and to improve menstrual symptoms. It contains ethinylestradiol, an estrogen, and etonogestrel, a progestin. It is used by insertion into the vagina. Pregnancy occurs in about 0.3% of women with perfect use and 9% of women with typical use.
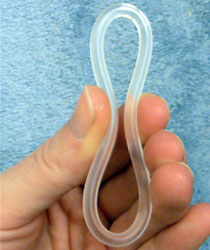
Combined hormonal contraception (CHC), or combined birth control, is a form of hormonal contraception which combines both an estrogen and a progestogen in varying formulations.

Cymegesolate, also known as cypionyl megestrol acetate or as megestrol acetate 3β-cypionate, is a progestin medication which was never marketed. It was developed in China in the late 1970s and early to mid 1980s for use as a hormonal contraceptive. The medication was formulated at a dose of 50–100 mg in combination with a "trace" dose of 0.25–0.5 mg quinestrol as a long-lasting, once-a-month combined oral contraceptive pill. This combination has been studied in 1,213 women across a total of 9,651 menstrual cycles, with contraceptive effectiveness of over 99.13% and "very few side effects." At the high dose, it showed an anovulation rate of only about 60%, and instead mediated its contraceptive effects via a marked anti-implantation effect.
Menstrual suppression refers to the practice of using hormonal management to stop or reduce menstrual bleeding. In contrast to surgical options for this purpose, such as hysterectomy or endometrial ablation, hormonal methods to manipulate menstruation are reversible.
References
- ↑ Wright KP, Johnson JV (October 2008). "Evaluation of extended and continuous use oral contraceptives". Therapeutics and Clinical Risk Management. 4 (5): 905–11. doi: 10.2147/tcrm.s2143 . PMC 2621397 . PMID 19209272.
- ↑ "Health Matters: Understanding Menstrual Suppression". Association of Reproductive Health Professionals. October 2006. Retrieved 2007-11-16.
- ↑ Contraceptive technology. Hatcher, Robert A. (Robert Anthony), 1937- (20th rev. ed.). New York, N.Y.: Ardent Media. 2011. p. 257. ISBN 978-1597080026. OCLC 244395421.
{{cite book}}: CS1 maint: others (link) - ↑ Organon (September 15, 2005). "NuvaRing is effective and well tolerated in extended use - Most women would like to decrease their number of periods a year". Archived from the original on December 12, 2007. Retrieved 2008-05-04.
Miller L, Verhoeven CH, Hout J (2005). "Extended regimens of the contraceptive vaginal ring: a randomized trial". Obstet Gynecol. 106 (3): 473–82. doi:10.1097/01.AOG.0000175144.08035.74. PMID 16135576. S2CID 46164922.
Barreiros FA, Guazzelli CA, de Araujo FF, Barbosa R (2007). "Bleeding patterns of women using extended regimens of the contraceptive vaginal ring". Contraception. 75 (3): 204–8. doi:10.1016/j.contraception.2006.10.009. PMID 17303490. - ↑ Stewart FH, Kaunitz AM, Laguardia KD, Karvois DL, Fisher AC, Friedman AJ (June 2005). "Extended use of transdermal norelgestromin/ethinyl estradiol: a randomized trial". Obstet Gynecol. 105 (6): 1389–96. doi:10.1097/01.AOG.0000160430.61799.f6. PMID 15932834. S2CID 8831803.
- ↑ "Lunelle (Monthly Injection)". Feminist Women's Health Center. January 2006. Archived from the original on 2007-05-29. Retrieved 2007-06-17.
- ↑ Rowlands S (October 2007). "Contraception and abortion". J R Soc Med. 100 (10): 465–8. doi:10.1177/014107680710001015. PMC 1997258 . PMID 17911129.
- ↑ Gladwell M (2000-03-10). "John Rock's Error". The New Yorker. Archived from the original on 2013-12-03. Retrieved 2016-02-17.
- ↑ Cooper DB, Patel P, Mahdy H (2022), "Oral Contraceptive Pills", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 28613632 , retrieved 2022-11-15
- ↑ Kripke C (2006). "Cyclic vs. continuous or extended-cycle combined contraceptives". American Family Physician. 73 (5): 804. PMID 16529087. Archived from the original on 2007-09-29. Retrieved 2007-06-17.
- 1 2 3 Edelman A, Micks E, Gallo MF, Jensen JT, Grimes DA (2014-07-29). "Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception". The Cochrane Database of Systematic Reviews. 2014 (7): CD004695. doi:10.1002/14651858.CD004695.pub3. ISSN 1469-493X. PMC 6837850 . PMID 25072731.
- ↑ Coffee AL, Kuehl TJ, Willis S, Sulak PJ (2006). "Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen". Am. J. Obstet. Gynecol. 195 (5): 1311–9. doi:10.1016/j.ajog.2006.05.012. PMID 16796986.
- ↑ Gerschultz KL, Sucato GS, Hennon TR, Murray PJ, Gold MA (2007). "Extended cycling of combined hormonal contraceptives in adolescents: physician views and prescribing practices". The Journal of Adolescent Health. 40 (2): 151–7. doi:10.1016/j.jadohealth.2006.09.013. PMID 17259055.
- ↑ "Menstruation Is Not A Disease". Society for Menstrual Cycle Research. 2007-06-08. Archived from the original on 2007-10-12. Retrieved 2007-11-16.
- ↑ Arowojolu AO, Gallo MF, Grimes DA, Garner SE (2004-07-19), Arowojolu A (ed.), "Combined oral contraceptive pills for treatment of acne", Cochrane Database of Systematic Reviews (3), Chichester, UK: John Wiley & Sons, Ltd: CD004425, doi:10.1002/14651858.cd004425.pub2, PMID 15266533 , retrieved 2022-11-15
- ↑ Montero-Vilchez T, Valenzuela-Amigo A, Cuenca-Barrales C, Arias-Santiago S, Leyva-García A, Molina-Leyva A (2021-07-15). "The Role of Oral Contraceptive Pills in Hidradenitis Suppurativa: A Cohort Study". Life. 11 (7): 697. Bibcode:2021Life...11..697M. doi: 10.3390/life11070697 . ISSN 2075-1729. PMC 8307628 . PMID 34357069.
- ↑ Wong CL, Farquhar C, Roberts H (2001-04-23), The Cochrane Collaboration (ed.), "Oral contraceptive pills for primary dysmenorrhoea", Cochrane Database of Systematic Reviews (4), Chichester, UK: John Wiley & Sons, Ltd: CD002120, doi:10.1002/14651858.CD002120, PMID 11687142 , retrieved 2022-11-16
- 1 2 Anderson FD, Gibbons W, Portman D (2006). "Long-term safety of an extended-cycle oral contraceptive (Seasonale): a 2-year multicenter open-label extension trial". Am. J. Obstet. Gynecol. 195 (1): 92–6. doi:10.1016/j.ajog.2005.12.045. PMID 16813747.
- ↑ Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD (2006). "Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results". Contraception. 74 (6): 439–45. doi:10.1016/j.contraception.2006.07.005. PMID 17157099.
- ↑ Fraser IS, Jansen RP (1 June 1983). "Why do inadvertent pregnancies occur in oral contraceptive users?". Contraception. 27 (6): 531–551. doi:10.1016/0010-7824(83)90019-7. PMID 6413129.
- ↑ Riechman SE, Lee CW (November 2022). "Oral Contraceptive Use Impairs Muscle Gains in Young Women". Journal of Strength and Conditioning Research. 36 (11): 3074–3080. doi:10.1519/JSC.0000000000004059. ISSN 1064-8011. PMID 33993156. S2CID 81112709.
- ↑ Krauss RM, Burkman RT (1 October 1992). "The metabolic impact of oral contraceptives". American Journal of Obstetrics and Gynecology. 167 (4): 1177–1184. doi:10.1016/s0002-9378(12)90408-1. ISSN 0002-9378. PMID 1415443.
- ↑ Simmons KB, Haddad LB, Nanda K, Curtis KM (1 January 2018). "Drug interactions between non-rifamycin antibiotics and hormonal contraception: a systematic review". American Journal of Obstetrics and Gynecology. 218 (1): 88–97.e14. doi:10.1016/j.ajog.2017.07.003. PMID 28694152. S2CID 36567820.
- ↑ "Seasonale (levonorgestrel/ethinyl estradiol) Tablets MACMIS ID #12748: 2004" (PDF). United States Department of Health & Human Services. December 2003. Archived from the original (PDF) on 2006-10-14. Retrieved 2006-10-22.
- 1 2 "Seasonale Extended Cycle OC Approved". About.com. February 2003. Archived from the original on 2008-02-03. Retrieved 2006-10-22.
- ↑ "FDA Approves Seasonale Oral Contraceptive". United States Food and Drug Administration. September 5, 2003. Archived from the original on 2006-10-07. Retrieved 2006-10-22.
- ↑ Magnan, Michelle, 2007-07-06, Health Canada approves Seasonale Archived 2014-05-25 at the Wayback Machine , Calgary Herald.
- ↑ "Paladin Labs Announces SEASONALE Launch". Paladin Labs, Inc. 2008-01-04. Archived from the original on 2013-01-19. Retrieved 2008-02-06.
- 1 2 "FDA Approves Lybrel, First Low Dose Combination Oral Contraceptive Offering Women the Opportunity to Be Period-Free Over Time". PR Newswire Association. 2007-05-22. Archived from the original on May 25, 2007. Retrieved 2007-06-17.