The combined oral contraceptive pill (COCP), often referred to as the birth control pill or colloquially as "the pill", is a type of birth control that is designed to be taken orally by women. It is the oral form of combined hormonal contraception. The pill contains two important hormones: a progestin and estrogen. When taken correctly, it alters the menstrual cycle to eliminate ovulation and prevent pregnancy.

Ethinylestradiol (EE) is an estrogen medication which is used widely in birth control pills in combination with progestins. In the past, EE was widely used for various indications such as the treatment of menopausal symptoms, gynecological disorders, and certain hormone-sensitive cancers. It is usually taken by mouth but is also used as a patch and vaginal ring.
Extended or continuous cycle combined oral contraceptive pills are a packaging of combined oral contraceptive pills (COCPs) that reduce or eliminate the withdrawal bleeding that would occur once every 28 days in traditionally packaged COCPs. It works by reducing the frequency of the pill-free or placebo days. Extended cycle use of COCPs may also be called menstrual suppression, although other hormonal medications or medication delivery systems may also be used to suppress menses. Any brand of combined oral contraceptive pills can be used in an extended or continuous manner by simply discarding the placebo pills; this is most commonly done with monophasic pills in which all of the pills in a package contain the same fixed dosing of a synthetic estrogen and a progestin in each active pill.
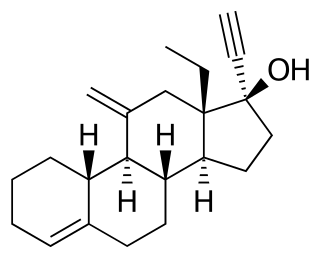
Desogestrel is a progestin medication which is used in birth control pills for women. It is also used in the treatment of menopausal symptoms in women. The medication is available and used alone or in combination with an estrogen. It is taken by mouth.

Drospirenone is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses. It is available both alone under the brand name Slynd and in combination with an estrogen under the brand name Yasmin among others. The medication is an analog of the drug spironolactone. Drospirenone is taken by mouth.
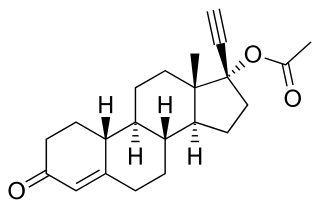
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is ingested orally.

Norelgestromin, or norelgestromine, sold under the brand names Evra and Ortho Evra among others, is a progestin medication which is used as a method of birth control for women. The medication is available in combination with an estrogen and is not available alone. It is used as a patch that is applied to the skin.

Norgestimate, sold under the brand names Ortho Tri-Cyclen and Previfem among others, is a progestin medication which is used in birth control pills for women and in menopausal hormone therapy. The medication is available in combination with an estrogen and is not available alone. It is taken by mouth.

Gestodene, sold under the brand names Femodene and Minulet among others, is a progestin medication which is used in birth control pills for women. It is also used in menopausal hormone therapy. The medication is available almost exclusively in combination with an estrogen. It is taken by mouth.

Mestranol, sold under the brand names Enovid, Norinyl, and Ortho-Novum among others, is an estrogen medication which has been used in birth control pills, menopausal hormone therapy, and the treatment of menstrual disorders. It is formulated in combination with a progestin and is not available alone. It is taken by mouth.
Birth control pills come in a variety of formulations. The main division is between combined oral contraceptive pills, containing both estrogens and synthetic progestogens (progestins), and progestogen only pills. Combined oral contraceptive pills also come in varying types, including varying doses of estrogen, and whether the dose of estrogen or progestogen changes from week to week.

Drospirenone/ethinylestradiol/levomefolic acid (EE/DRSP/LMF), sold under the brand name Beyaz among others, is a combination of ethinylestradiol (EE), an estrogen, drospirenone (DRSP), a progestogen, antimineralocorticoid, and antiandrogen, and levomefolic acid (LMF), a form of vitamin B9, which is used as a birth control pill to prevent pregnancy in women. The formulation contains folate as the calcium salt of levomefolic acid to lower the risk of complications such as fetal neural tube defects should the medication fail as a form of birth control. EE/DRSP/LMF was approved for use by the US Food and Drug Administration (FDA) in September 2010.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.
Combined birth control pills that contain natural estradiol or an estradiol ester include:

Estradiol (E2) is a medication and naturally occurring steroid hormone. It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women. It is also used in hormonal birth control for women, in feminizing hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses. Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.
Norelgestromin/ethinylestradiol, sold under the brand name Ortho Evra among others, is a contraceptive patch containing the progestin norelgestromin and the estrogen ethinylestradiol.

Ethinylestradiol/etonogestrel, sold under the brand names NuvaRing among others, is a hormonal vaginal ring used for birth control and to improve menstrual symptoms. It contains ethinylestradiol, an estrogen, and etonogestrel, a progestin. It is used by insertion into the vagina. Pregnancy occurs in about 0.3% of women with perfect use and 9% of women with typical use.
Combined hormonal contraception (CHC), or combined birth control, is a form of hormonal contraception which combines both an estrogen and a progestogen in varying formulations.

Ethinylestradiol/cyproterone acetate (EE/CPA), also known as co-cyprindiol and sold under the brand names Diane and Diane-35 among others, is a combination of ethinylestradiol (EE), an estrogen, and cyproterone acetate (CPA), a progestin and antiandrogen, which is used as a birth control pill to prevent pregnancy in women. It is also used to treat androgen-dependent conditions in women such as acne, seborrhea, excessive facial/body hair growth, scalp hair loss, and high androgen levels associated with ovaries with cysts. The medication is taken by mouth once daily for 21 days, followed by a 7-day free interval.