Related Research Articles

Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity (electrowinning).
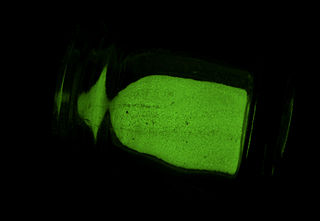
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam in a cathode-ray tube.

Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main source of iron for the steel industry. Fe2O3 is readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition; however, in chemistry, rust is considered an ill-defined material, described as Hydrous ferric oxide.

Titanium dioxide, also known as titanium(IV) oxide or titania, is the inorganic compound with the chemical formula TiO
2. When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white, water-insoluble solid, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at $13.2 billion.

Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically.

Zinc sulfide is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics.

Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
Lithopone, C.I. Pigment White 5, is a mixture of inorganic compounds, widely used as a white pigment powder. It is composed of a mixture of barium sulfate and zinc sulfide. These insoluble compounds blend well with organic compounds and confer opacity. It was made popular by the cheap production costs, greater coverage. Related white pigments include titanium dioxide, zinc oxide, zinc sulfide, and white lead.

Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite.

In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity depends on the ability of the catalyst to create electron–hole pairs, which generate free radicals (e.g. hydroxyl radicals: •OH) able to undergo secondary reactions. Its practical application was made possible by the discovery of water electrolysis by means of titanium dioxide (TiO2).

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.

A ferrite is a ceramic material made by mixing and firing large proportions of iron(III) oxide (Fe2O3, rust) blended with small proportions of one or more additional metallic elements, such as strontium, barium, manganese, nickel, and zinc. They are ferrimagnetic, meaning they can be magnetized or attracted to a magnet. Unlike other ferromagnetic materials, most ferrites are not electrically conductive, making them useful in applications like magnetic cores for transformers to suppress eddy currents. Ferrites can be divided into two families based on their resistance to being demagnetized (magnetic coercivity).

Zinc nitrate is an inorganic chemical compound with the formula Zn(NO3)2. This colorless, crystalline salt is highly deliquescent. It is typically encountered as a hexahydrate Zn(NO3)2·6H2O. It is soluble in both water and alcohol.

Zinc nitride (Zn3N2) is an inorganic compound of zinc and nitrogen, usually obtained as (blue)grey crystals. It is a semiconductor. In pure form, it has the anti-bixbyite structure.
Cobalt(II) oxide is an inorganic compound that has been described as an olive-green or gray solid. It is used extensively in the ceramics industry as an additive to create blue colored glazes and enamels as well as in the chemical industry for producing cobalt(II) salts. A related material is cobalt(II,III) oxide, a black solid with the formula Co3O4.

Chelates in animal feed is jargon for metalloorganic compounds added to animal feed. The compounds provide sources of various metals that improve the health or marketability of the animal. Typical metals salts are derived from cobalt, copper, iron, manganese, and zinc. The objective of supplementation with trace minerals is to avoid a variety of deficiency diseases. Trace minerals carry out key functions in relation to many metabolic processes, most notably as cofactors for enzymes and hormones, and are essential for optimum health, growth and productivity. For example, supplementary minerals help ensure good growth, bone development, feathering in birds, hoof, skin and hair quality in mammals, enzyme structure and functions, and appetite. Deficiency of trace minerals affect many metabolic processes and so may be manifested by different symptoms, such as poor growth and appetite, reproductive failures, impaired immune responses, and general ill-thrift. From the 1950s to the 1990s most trace mineral supplementation of animal diets was in the form of inorganic minerals, and these largely eradicated associated deficiency diseases in farm animals. The role in fertility and reproductive diseases of dairy cattle highlights that organic forms of Zn are retained better than inorganic sources and so may provide greater benefit in disease prevention, notably mastitis and lameness.
Compounds of zinc are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript behavior, they are generally colorless, do not readily engage in redox reactions, and generally adopt symmetrical structures.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).
Evolution of metal ions in biological systems refers to the incorporation of metallic ions into living organisms and how it has changed over time. Metal ions have been associated with biological systems for billions of years, but only in the last century have scientists began to truly appreciate the scale of their influence. Major and minor metal ions have become aligned with living organisms through the interplay of biogeochemical weathering and metabolic pathways involving the products of that weathering. The associated complexes have evolved over time.
References
- ↑ Völz, Hans G. et al. "Pigments, Inorganic" in Ullmann's Encyclopedia of Industrial Chemistry, 2006 Wiley-VCH, Weinheim. doi : 10.1002/14356007.a20_243.pub2.
- ↑ F. Wagenknecht, and R. Juza "Rinmann's Green" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1092.
- ↑ Djerdj, Igor; Jaglicic, Zvonko; Arcon, Denis; Niederberger, Markus (2010). "Co-Doped ZnO nanoparticles: Minireview". Nanoscale. 2 (7): 1096–1104. Bibcode:2010Nanos...2.1096D. doi:10.1039/c0nr00148a. PMID 20648333.