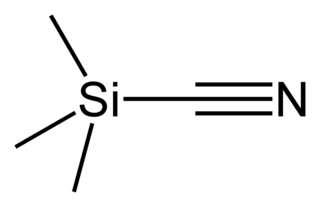Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.

Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2. It forms hydrates. Zinc chloride, anhydrous and its hydrates are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride. This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
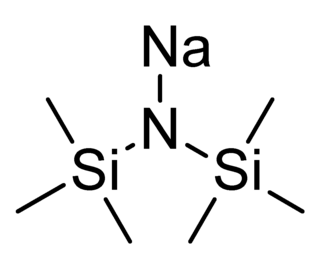
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula NaN(Si 3)2. This species, usually called NaHMDS, is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are that it is commercially available as a solid and it is soluble not only in ethers, such as THF or diethyl ether, but also in aromatic solvents, like benzene and toluene by virtue of the lipophilic TMS groups.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water.

Di-tert-butyl peroxide or DTBP is an organic compound consisting of a peroxide group bonded to two tert-butyl groups. It is one of the most stable organic peroxides, due to the tert-butyl groups being bulky. It is a colorless liquid.

Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium compound. The DMG interacts with lithium through a hetero atom. Examples of DMG's are the methoxy group, a tertiary amine group and an amide group.The compound can be produced by directed lithiation of anisole.
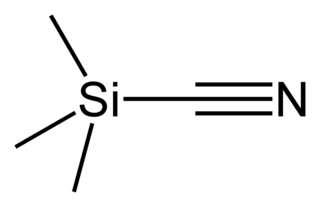
Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide. It is prepared by the reaction of lithium cyanide and trimethylsilyl chloride:

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
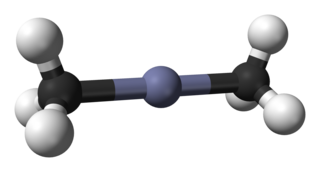
Dimethylzinc, also known as zinc methyl, DMZ, or DMZn, is an organozinc compound with the chemical formula Zn(CH3)2. It belongs to the large series of similar compounds such as diethylzinc.
Zinc–coppercouple is an alloy of zinc and copper that is employed as a reagent in organic synthesis. The “couple” was popularized after the report by Simmons and Smith in 1959 of its application as an activated source of zinc required for formation of an organozinc reagent in the Simmons–Smith cyclopropanation of alkenes. The couple has been widely applied as a reagent in other reactions requiring activated zinc metal. Zinc–copper couple does not refer to a rigorously defined chemical structure or alloy composition. The couple may contain varying proportions of copper and zinc; the zinc content is typically greater than 90%, although an alloy containing similar proportions of zinc and copper is used in some cases. The couple is frequently prepared as a darkly-colored powder and is slurried in an ethereal solvent prior to being used in slight excess relative to the substrate. Activation of zinc by copper is essential to the couple’s utility, but the origin of this effect is poorly documented. It is speculated that copper enhances reactivity of zinc at the surface of the alloy.
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript appearance and behavior: they are generally colorless, do not readily engage in redox reactions, and generally adopt symmetrical structures.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
Organorhenium chemistry describes the compounds with Re−C bonds. Because rhenium is a rare element, relatively few applications exist, but the area has been a rich source of concepts and a few useful catalysts.

tert-Butylthiol, also known as 2-methylpropane-2-thiol, 2-methyl-2-propanethiol, tert-butyl mercaptan (TBM), and t-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol is used as an odorant for natural gas, which is otherwise odorless. It may also have been used as a flavoring agent.

Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications.
Di-tert-butyl-iminodicarboxylate is an organic compound that can be described with the formula [(CH3)3COC(O)]2NH. It is a white solid that is soluble in organic solvents. The compound is used as a reagent for the preparation of primary amines from alkyl halides. It was popularized as an alternative to the Gabriel synthesis for the same conversion. Amines can also be prepared from alcohols by dehydration using the Mitsunobu reaction.

tert-Butoxybis(dimethylamino)methane is an organic compound with the formula (CH3)3COCH(N(CH3)2)2. The compound is classified as an aminal ester, i.e. the tert-butyl alcohol derivative of the aminal bis(dimethylamino)methane. It is a colorless liquid with a amine odor.