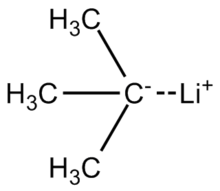
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure RC≡CM. Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.
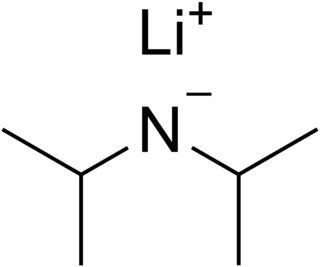
Lithium diisopropylamide is a chemical compound with the molecular formula LiN(CH 2)2. It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group.

In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.

n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of organolithium reagent. The reaction was discovered by Robert H. Shapiro in 1967. The Shapiro reaction was used in the Nicolaou Taxol total synthesis. This reaction is very similar to the Bamford–Stevens reaction, which also involves the basic decomposition of tosyl hydrazones.

Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses. Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute.

Hydroperoxides or peroxols are compounds of the form ROOH, which contain the hydroperoxy functional group (–OOH). The hydroperoxide anion and the neutral hydroperoxyl radical (HOO·) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.

A Grignard reagent or Grignard compound is a chemical compound with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

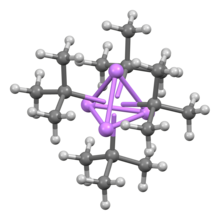
Methyllithium is the simplest organolithium reagent, with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.

sec-Butyllithium is an organometallic compound with the formula CH3CHLiCH2CH3, abbreviated sec-BuLi or s-BuLi. This chiral organolithium reagent is used as a source of sec-butyl carbanion in organic synthesis.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
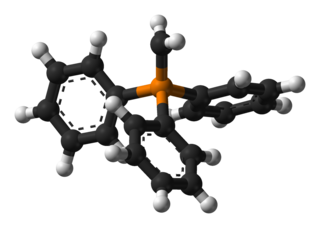
Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species.

Vinyllithium is an organolithium compound with the formula LiC2H3. A colorless or white solid, it is encountered mainly as a solution in tetrahydrofuran (THF). It is a reagent in synthesis of organic compounds, especially for vinylations.
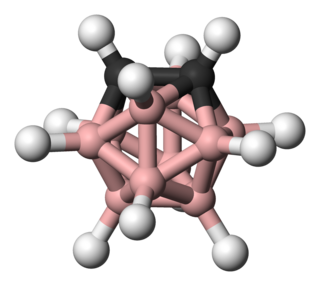
ortho-Carborane is the organoboron compound with the formula C2B10H12. The prefix ortho is derived from ortho. It is the most prominent carborane. This derivative has been considered for a wide range of applications from heat-resistant polymers to medical applications. It is a colorless solid that melts, without decomposition, at 320 °C.
In organometallic chemistry, metal–halogen exchange is a fundamental reaction that converts an organic halide into an organometallic product. The reaction commonly involves the use of electropositive metals and organochlorides, bromides, and iodides. Particularly well-developed is the use of metal–halogen exchange for the preparation of organolithium compounds.

(Trimethylsilyl)methyllithium is classified both as an organolithium compound and an organosilicon compound. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2tms. It crystallizes as the hexagonal prismatic hexamer [LiCH2tms]6, akin to some polymorphs of methyllithium. Many adducts have been characterized including the diethyl ether complexed cubane [Li4(μ3-CH2tms)4(Et2O)2] and [Li2(μ-CH2tms)2(tmeda)2].

Sodium acetylacetonate is an organic compound with the nominal formula Na[CH(C(O)CH3)2]. This white, water-soluble solid is the conjugate base of acetylacetone.