Related Research Articles

Niobium is a chemical element with chemical symbol Nb and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has similar ductility to iron. Niobium oxidizes in Earth's atmosphere very slowly, hence its application in jewelry as a hypoallergenic alternative to nickel. Niobium is often found in the minerals pyrochlore and columbite, hence the former name "columbium". Its name comes from Greek mythology: Niobe, daughter of Tantalus, the namesake of tantalum. The name reflects the great similarity between the two elements in their physical and chemical properties, which makes them difficult to distinguish.

Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered, even down to near absolute zero, a superconductor has a characteristic critical temperature below which the resistance drops abruptly to zero. An electric current through a loop of superconducting wire can persist indefinitely with no power source.

High-temperature superconductors are defined as materials that behave as superconductors at temperatures above 77 K, the boiling point of liquid nitrogen. The adjective "high temperature" is only in respect to previously known superconductors, which function at even colder temperatures close to absolute zero. In absolute terms, these "high temperatures" are still far below ambient, and therefore require cooling. The first high-temperature superconductor was discovered in 1986, by IBM researchers Bednorz and Müller, who were awarded the Nobel Prize in Physics in 1987 "for their important break-through in the discovery of superconductivity in ceramic materials". Most high-Tc materials are type-II superconductors.
Metallic hydrogen is a phase of hydrogen in which it behaves like an electrical conductor. This phase was predicted in 1935 on theoretical grounds by Eugene Wigner and Hillard Bell Huntington.

A lithium-ion or Li-ion battery is a type of rechargeable battery which uses the reversible reduction of lithium ions to store energy. The negative electrode of a conventional lithium-ion cell is typically graphite, a form of carbon. This negative electrode is sometimes called the anode as it acts as an anode during discharge. The positive electrode is typically a metal oxide; the positive electrode is sometimes called the cathode as it acts as a cathode during discharge. Positive and negative electrodes remain positive and negative in normal use whether charging or discharging and are therefore clearer terms to use than anode and cathode which are reversed during charging.
Palladium hydride is metallic palladium that contains a substantial quantity of hydrogen within its crystal lattice. Despite its name, it is not an ionic hydride but rather an alloy of palladium with metallic hydrogen that can be written PdHx. At room temperature, palladium hydrides may contain two crystalline phases, α and β. Pure α-phase exists at x < 0.017 whereas pure β-phase is realised for x > 0.58; intermediate x values correspond to α-β mixtures.
A room-temperature superconductor is a material that is capable of exhibiting superconductivity at operating temperatures above 0 °C, that is, temperatures that can be reached and easily maintained in an everyday environment. As of 2020, the material with the highest claimed superconducting temperature is an extremely pressurized carbonaceous sulfur hydride with a critical transition temperature of +15 °C at 267 GPa. On 22 September 2022, the original article reporting superconductivity in the carbonaceous sulfur hydride material was retracted by Nature journal editorial board due to a non standard, user-defined data analysis, calling into question the scientific validity of the claim.

Superconductivity is the phenomenon of certain materials exhibiting zero electrical resistance and the expulsion of magnetic fields below a characteristic temperature. The history of superconductivity began with Dutch physicist Heike Kamerlingh Onnes's discovery of superconductivity in mercury in 1911. Since then, many other superconducting materials have been discovered and the theory of superconductivity has been developed. These subjects remain active areas of study in the field of condensed matter physics.

Lithium nitride is a compound with the formula Li3N. It is the only stable alkali metal nitride. The solid has a reddish-pink color and high melting point.

Graphite intercalation compounds are complex materials having a formula CXm where the ion Xn+ or Xn− is inserted (intercalated) between the oppositely charged carbon layers. Typically m is much less than 1. These materials are deeply colored solids that exhibit a range of electrical and redox properties of potential applications.
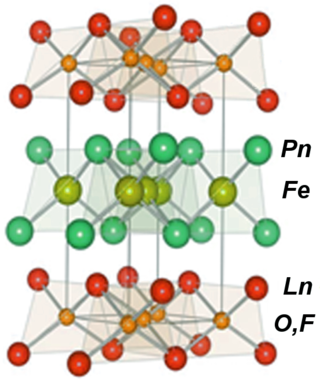
Iron-based superconductors (FeSC) are iron-containing chemical compounds whose superconducting properties were discovered in 2006. In 2008, led by recently discovered iron pnictide compounds, they were in the first stages of experimentation and implementation..
In chemistry, oxypnictides are a class of materials composed of oxygen, a pnictogen and one or more other elements. Although this group of compounds has been recognized since 1995, interest in these compounds increased dramatically after the publication of the superconducting properties of LaOFeP and LaOFeAs which were discovered in 2006 and 2008. In these experiments the oxide was partly replaced by fluoride.
A solid-state battery deploys solid-state technology using solid electrodes and a solid electrolyte, instead of the liquid or polymer gel electrolytes found in lithium-ion or lithium polymer batteries.

Binary compounds of silicon are binary chemical compounds containing silicon and one other chemical element. Technically the term silicide is reserved for any compounds containing silicon bonded to a more electropositive element. Binary silicon compounds can be grouped into several classes. Saltlike silicides are formed with the electropositive s-block metals. Covalent silicides and silicon compounds occur with hydrogen and the elements in groups 10 to 17.

Jean-Marie Tarascon FRSC is Professor of Chemistry at the Collège de France in Paris and Director of the French Research Network on Electrochemical Energy Storage (RS2E).
Lithium monoxide anion (LiO−) is a superbase existing in the gas phase. It was the strongest known base until 2008, when the isomeric diethynylbenzene dianions were determined to have a higher proton affinity. The methanide ion CH3− was the strongest known base before lithium monoxide anion was discovered.
A polyhydride or superhydride is a compound that contains an abnormally large amount of hydrogen. This can be described as high hydrogen stoichiometry. Examples include iron pentahydride FeH5, LiH6, and LiH7. By contrast, the more well known lithium hydride only has one hydrogen atom.
In chemistry, a hydridonitride is a chemical compound that contains hydride and nitride ions in a single phase. These inorganic compounds are distinct from inorganic amides and imides as the hydrogen does not share a bond with nitrogen, and contain a larger proportion of metals.
A chloride nitride is a mixed anion compound containing both chloride (Cl−) and nitride ions (N3−). Another name is metallochloronitrides. They are a subclass of halide nitrides or pnictide halides.
References
- 1 2 Feng, Ji; Hennig, Richard G.; Ashcroft, N. W.; Hoffmann, Roald (2008). "Emergent reduction of electronic state dimensionality in dense ordered Li-Be alloys". Nature. 451 (7177): 445–8. Bibcode:2008Natur.451..445F. doi: 10.1038/nature06442 . PMID 18216850.
- ↑ Errea, Ion; Martinez-Canales, Miguel; Bergara, Aitor (2008). "Ab initio study of superconducting hexagonal Be2Li under pressure". Physical Review B. 78 (17): 172501. Bibcode:2008PhRvB..78q2501E. doi:10.1103/PhysRevB.78.172501.
- ↑ Xu, Ying; Chen, Changbo; Wu, Baojia (2012). "Superconductivity in ordered LiBe alloy under high pressure: A first-principles study". Solid State Communications. 152 (2): 151. Bibcode:2012SSCom.152..151X. doi:10.1016/j.ssc.2011.09.032.