
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow solid. It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications. Its vivid yellow color led to its adoption as a pigment for the yellow paint "cadmium yellow" in the 18th century.

Lithium hydride is an inorganic compound with the formula LiH. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not soluble but reactive with all protic organic solvents. It is soluble and nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molar mass of 7.95 g/mol, it is the lightest ionic compound.
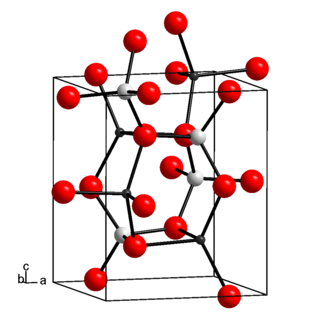
Lithium aluminate, also called lithium aluminium oxide, is an inorganic chemical compound, an aluminate of lithium. In microelectronics, lithium aluminate is considered as a lattice matching substrate for gallium nitride. In nuclear technology, lithium aluminate is of interest as a solid tritium breeder material, for preparing tritium fuel for nuclear fusion.
Lithium stearate is a chemical compound with the formula LiO2C(CH2)16CH3. It is formally classified as a soap (a salt of a fatty acid). Lithium stearate is a white soft solid, prepared by the reaction of lithium hydroxide and stearic acid.

Lithium cyanide is an inorganic compound with the chemical formula LiCN. It is a toxic, white colored, hygroscopic, water-soluble salt that finds only niche uses.
Lithium molybdenum purple bronze is a chemical compound with formula Li
0.9Mo
6O
17, that is, a mixed oxide of molybdenum and lithium. It can be obtained as flat crystals with a purple-red color and metallic sheen.

Promethium(III) fluoride or promethium trifluoride is a salt of promethium and fluorine with the formula PmF3.
Lithium phosphide is an inorganic compound of lithium and phosphorus with the chemical formula Li
3P.
Lithium arsenide is a binary inorganic compound of lithium and arsenic with the chemical formula LiAs.

Lithium lactate is a chemical compound, a salt of lithium and lactic acid with the formula CH3CH(OH)COOLi, an amorphous solid, very soluble in water.
Silver laurate is an inorganic compound, a salt of silver and lauric acid with the formula AgC
11H
23COO, colorless (white) crystals.
Lead(II) laurate is an metal-organic compound with the chemical formula Pb(O2C 10CH3)2. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid. Like most soaps, it does not dissolve in water. Lead soaps have been used as stabilizers and plasticizers in PVC.
Copper(II) laurate is an metal-organic compound with the chemical formula Cu(C
11H
23COO)
2. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Cobalt laurate is an metal-organic compound with the chemical formula C
24H
48CoO
4. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Nickel(II) laurate is an metal-organic compound with the chemical formula C
24H
46NiO
4. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Zinc laurate is an metal-organic compound with the chemical formula C
24H
46ZnO
4. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Manganese laurate is an metal-organic compound with the chemical formula C
24H
48MnO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Aluminum laurate is an metal-organic compound with the chemical formula C
36H
69AlO
6. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Magnesium laurate is a metal-organic compound with the chemical formula C
24H
46MgO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Potassium laurate is a metal-organic compound with the chemical formula C
12H
23KO
2. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.





