
Piezoelectricity is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure and latent heat. It is derived from Ancient Greek πιέζω (piézō) 'to squeeze or press', and ἤλεκτρον (ḗlektron) 'amber'.
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are also piezoelectric and pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Joseph Valasek. Thus, the prefix ferro, meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric and ferromagnetic are known as multiferroics.

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.
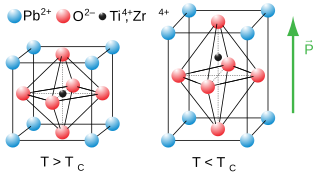
Lead zirconate titanate, also called lead zirconium titanate and commonly abbreviated as PZT, is an inorganic compound with the chemical formula Pb[ZrxTi1−x]O3(0 ≤ x ≤ 1). It is a ceramic perovskite material that shows a marked piezoelectric effect, meaning that the compound changes shape when an electric field is applied. It is used in a number of practical applications such as ultrasonic transducers and piezoelectric resonators. It is a white to off-white solid.
Electroceramics are a class of ceramic materials used primarily for their electrical properties.
In chemistry, a mixed oxide is a somewhat informal name for an oxide that contains cations of more than one chemical element or cations of a single element in several states of oxidation.

Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric, pyroelectric, and piezoelectric ceramic material that exhibits the photorefractive effect. It is used in capacitors, electromechanical transducers and nonlinear optics.
Lead scandium tantalate (PST) is a mixed oxide of lead, scandium, and tantalum. It has the formula Pb(Sc0.5Ta0.5)O3. It is a ceramic material with a perovskite structure, where the Sc and Ta atoms at the B site have an arrangement that is intermediate between ordered and disordered configurations, and can be fine-tuned with thermal treatment. It is ferroelectric at temperatures below 270 K (−3 °C; 26 °F), and is also piezoelectric. Like structurally similar lead zirconate titanate and barium strontium titanate, PST can be used for manufacture of uncooled focal plane array infrared imaging sensors for thermal cameras.
Lanthanum strontium cobalt ferrite (LSCF), also called lanthanum strontium cobaltite ferrite is a specific ceramic oxide derived from lanthanum cobaltite of the ferrite group. It is a phase containing lanthanum(III) oxide, strontium oxide, cobalt oxide and iron oxide with the formula La
xSr
1-xCo
yFe
1-yO
3, where 0.1≤x≤0.4 and 0.2≤y≤0.8.

Lanthanum strontium manganite (LSM or LSMO) is an oxide ceramic material with the general formula La1−xSrxMnO3, where x describes the doping level.
Cuprate superconductors are a family of high-temperature superconducting materials made of layers of copper oxides (CuO2) alternating with layers of other metal oxides, which act as charge reservoirs. At ambient pressure, cuprate superconductors are the highest temperature superconductors known. However, the mechanism by which superconductivity occurs is still not understood.
LSAT is the most common name for the inorganic compound lanthanum aluminate - strontium aluminium tantalate, which has the chemical formula (LaAlO3)0.3(Sr2TaAlO6)0.7 or its less common alternative: (La0.18Sr0.82)(Al0.59Ta0.41)O3. LSAT is a hard, optically transparent oxide of the elements lanthanum, aluminium, strontium and tantalum. LSAT has the perovskite crystal structure, and its most common use is as a single crystal substrate for the growth of epitaxial thin films.
Lanthanum aluminate is an inorganic compound with the formula LaAlO3, often abbreviated as LAO. It is an optically transparent ceramic oxide with a distorted perovskite structure.
Relaxor ferroelectrics are ferroelectric materials that exhibit high electrostriction. As of 2015, although they have been studied for over fifty years, the mechanism for this effect is still not completely understood, and is the subject of continuing research.
Sodium bismuth titanate or bismuth sodium titanium oxide (NBT or BNT) is a solid inorganic compound of sodium, bismuth, titanium and oxygen with the chemical formula of Na0.5Bi0.5TiO3 or Bi0.5Na0.5TiO3. This compound adopts the perovskite structure.
A piezoelectric microelectromechanical system (piezoMEMS) is a miniature or microscopic device that uses piezoelectricity to generate motion and carry out its tasks. It is a microelectromechanical system that takes advantage of an electrical potential that appears under mechanical stress. PiezoMEMS can be found in a variety of applications, such as switches, inkjet printer heads, sensors, micropumps, and energy harvesters.
A process related to the sol-gel route is the Pechini, or liquid mix, process. An aqueous solution of suitable oxides or salts is mixed with an alpha-hydroxycarboxylic acid such as citric acid. Chelation, or the formation of complex ring-shaped compounds around the metal cations, takes place in the solution. A polyhydroxy alcohol is then added, and the liquid is heated to 150–250 °C to allow the chelates to polymerize, or form large, cross-linked networks. As excess water is removed by heating, a solid polymeric resin results. Eventually, at still higher temperatures of 500–900 °C, the resin is decomposed or charred, and ultimately a mixed oxide is obtained. Particle size is extremely small, typically 20 to 50 nanometres, with intimate mixing taking place on the atomic scale.
Nickel niobate is a complex oxide which as a solid material has found potential applications in catalysis and lithium batteries.








