
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".

Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply.

Electrical discharge machining (EDM), also known as spark machining, spark eroding, die sinking, wire burning or wire erosion, is a metal fabrication process whereby a desired shape is obtained by using electrical discharges (sparks). Material is removed from the work piece by a series of rapidly recurring current discharges between two electrodes, separated by a dielectric liquid and subject to an electric voltage. One of the electrodes is called the tool-electrode, or simply the tool or electrode, while the other is called the workpiece-electrode, or work piece. The process depends upon the tool and work piece not making physical contact. Extremely hard materials like carbides, ceramics, titanium alloys and heat treated tool steels that are very difficult to machine using conventional machining can be precisely machined by EDM.

Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.

Machining is a manufacturing process whereby a desired shape or part is achieved by the controlled removal of material from a larger piece of raw material by cutting; it is most often performed with metal material. These processes are collectively called subtractive manufacturing, which utilizes machine tools, in contrast to additive manufacturing, which uses controlled addition of material.
Electropolishing, also known as electrochemical polishing, anodic polishing, or electrolytic polishing, is an electrochemical process that removes material from a metallic workpiece, reducing the surface roughness by levelling micro-peaks and valleys, improving the surface finish. Electropolishing is often compared to, but distinctly different from, electrochemical machining. It is used to polish, passivate, and deburr metal parts. It is often described as the reverse of electroplating. It may be used in lieu of abrasive fine polishing in microstructural preparation.
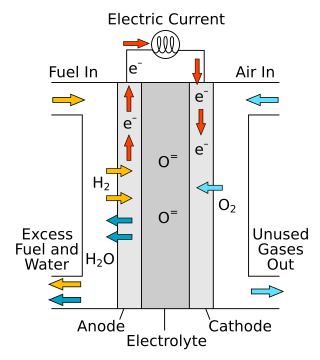
A solid oxide fuel cell is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte.

Surface finishing is a broad range of industrial processes that alter the surface of a manufactured item to achieve a certain property. Finishing processes may be employed to: improve appearance, adhesion or wettability, solderability, corrosion resistance, tarnish resistance, chemical resistance, wear resistance, hardness, modify electrical conductivity, remove burrs and other surface flaws, and control the surface friction. In limited cases some of these techniques can be used to restore original dimensions to salvage or repair an item. An unfinished surface is often called mill finish.
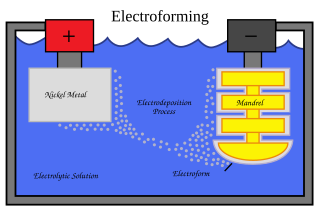
Electroforming is a metal forming process in which parts are fabricated through electrodeposition on a model, known in the industry as a mandrel. Conductive (metallic) mandrels are treated to create a mechanical parting layer, or are chemically passivated to limit electroform adhesion to the mandrel and thereby allow its subsequent separation. Non-conductive mandrels require the deposition of a conductive layer prior to electrodeposition. Such layers can be deposited chemically, or using vacuum deposition techniques. The outer surface of the mandrel forms the inner surface of the form.

A burr is a raised edge or small piece of material that remains attached to a workpiece after a modification process. It is usually an unwanted piece of material and is removed with a deburring tool in a process called deburring. Burrs are most commonly created by machining operations, such as grinding, drilling, milling, engraving or turning. It may be present in the form of a fine wire on the edge of a freshly sharpened tool or as a raised portion of a surface; this type of burr is commonly formed when a hammer strikes a surface. Deburring accounts for a significant portion of manufacturing costs.
A polymer-based battery uses organic materials instead of bulk metals to form a battery. Currently accepted metal-based batteries pose many challenges due to limited resources, negative environmental impact, and the approaching limit of progress. Redox active polymers are attractive options for electrodes in batteries due to their synthetic availability, high-capacity, flexibility, light weight, low cost, and low toxicity. Recent studies have explored how to increase efficiency and reduce challenges to push polymeric active materials further towards practicality in batteries. Many types of polymers are being explored, including conductive, non-conductive, and radical polymers. Batteries with a combination of electrodes are easier to test and compare to current metal-based batteries, however batteries with both a polymer cathode and anode are also a current research focus. Polymer-based batteries, including metal/polymer electrode combinations, should be distinguished from metal-polymer batteries, such as a lithium polymer battery, which most often involve a polymeric electrolyte, as opposed to polymeric active materials.
The programmable metallization cell, or PMC, is a non-volatile computer memory developed at Arizona State University. PMC, a technology developed to replace the widely used flash memory, providing a combination of longer lifetimes, lower power, and better memory density. Infineon Technologies, who licensed the technology in 2004, refers to it as conductive-bridging RAM, or CBRAM. CBRAM became a registered trademark of Adesto Technologies in 2011. NEC has a variant called "Nanobridge" and Sony calls their version "electrolytic memory".

Grinding is a type of abrasive machining process which uses a grinding wheel as cutting tool.
Electrochemical grinding is a process that removes electrically conductive material by grinding with a negatively charged abrasive grinding wheel, an electrolyte fluid, and a positively charged workpiece. Materials removed from the workpiece stay in the electrolyte fluid. Electrochemical grinding is similar to electrochemical machining but uses a wheel instead of a tool shaped like the contour of the workpiece.

The IsaKidd Technology is a copper electrorefining and electrowinning technology that was developed independently by Copper Refineries Proprietary Limited (“CRL”), a Townsville, Queensland, subsidiary of MIM Holdings Limited, and at the Falconbridge Limited (“Falconbridge”) now-dismantled Kidd Creek refinery that was at Timmins, Ontario. It is based around the use of reusable cathode starter sheets for copper electrorefining and the automated stripping of the deposited “cathode copper” from them.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.

Galvanic corrosion is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. This phenomenon is named after Italian physician Luigi Galvani (1737–1798).

Aluminum electrolytic capacitors are polarized electrolytic capacitors whose anode electrode (+) is made of a pure aluminum foil with an etched surface. The aluminum forms a very thin insulating layer of aluminum oxide by anodization that acts as the dielectric of the capacitor. A non-solid electrolyte covers the rough surface of the oxide layer, serving in principle as the second electrode (cathode) (-) of the capacitor. A second aluminum foil called "cathode foil" contacts the electrolyte and serves as the electrical connection to the negative terminal of the capacitor.

















