
Sodium hypochlorite is an alkaline inorganic chemical compound with the formula NaOCl. It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations and hypochlorite anions.
4-Aminobenzoic acid (also known as para-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the para position) is an organic compound with the formula H2NC6H4CO2H. PABA is a white solid, although commercial samples can appear gray. It is slightly soluble in water. It consists of a benzene ring substituted with amino and carboxyl groups. The compound occurs extensively in the natural world.

Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually handled as an aqueous solution. It is commonly used as a bleach. More recent developments have extended its applications in food processing and as a disinfectant.

Hypochlorous acid is an inorganic compound with the chemical formula ClOH, also written as HClO, HOCl, or ClHO. Its structure is H−O−Cl. It is an acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite anion, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor, chlorine.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is acutely poisonous.
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds containing at least one covalently bonded atom of chlorine. The chloroalkane class includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.

Sodium chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant.
Calcium hypochlorite is an inorganic compound with chemical formula Ca(ClO)2, also written as Ca(OCl)2. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical. It is the main active ingredient of commercial products called bleaching powder, used for water treatment and as a bleaching agent.

The Baker–Venkataraman rearrangement is the chemical reaction of 2-acetoxyacetophenones with base to form 1,3-diketones.

Azinphos-methyl (Guthion) is a broad spectrum organophosphate insecticide manufactured by Bayer CropScience, Gowan Co., and Makhteshim Agan. Like other pesticides in this class, it owes its insecticidal properties to the fact that it is an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
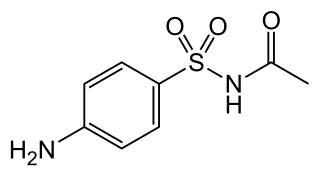
Sulfacetamide is a sulfonamide antibiotic.
The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.

Acifluorfen is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase which is necessary for chlorophyll synthesis. Soybeans naturally have a high tolerance to acifluorfen and its salts, via metabolic disposal by glutathione S-transferase. It is effective against broadleaf weeds and grasses and is used agriculturally on fields growing soybeans, peanuts, peas, and rice.
4-Hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors are a class of herbicides that prevent growth in plants by blocking 4-Hydroxyphenylpyruvate dioxygenase, an enzyme in plants that breaks down the amino acid tyrosine into molecules that are then used by plants to create other molecules that plants need. This process of breakdown, or catabolism, and making new molecules from the results, or biosynthesis, is something all living things do. HPPD inhibitors were first brought to market in 1980, although their mechanism of action was not understood until the late 1990s. They were originally used primarily in Japan in rice production, but since the late 1990s have been used in Europe and North America for corn, soybeans, and cereals, and since the 2000s have become more important as weeds have become resistant to glyphosate and other herbicides. Genetically modified crops are under development that include resistance to HPPD inhibitors. There is a pharmaceutical drug on the market, nitisinone, that was originally under development as an herbicide as a member of this class, and is used to treat an orphan disease, type I tyrosinemia.

Hydroxylamine-O-sulfonic acid (HOSA) or aminosulfuric acid is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum. It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles.

Chlorine-releasing compounds, also known as chlorine base compounds, is jargon to describe certain chlorine-containing substances that are used as disinfectants and bleaches. They include the following chemicals: sodium hypochlorite, chloramine, halazone, and sodium dichloroisocyanurate. They are widely used to disinfect water and medical equipment, and surface areas as well as bleaching materials such as cloth. The presence of organic matter can make them less effective as disinfectants. They come as a liquid solution, or as a powder that is mixed with water before use.

DMTMM is an organic triazine derivative commonly used for activation of carboxylic acids, particularly for amide synthesis. Amide coupling is one of the most common reactions in organic chemistry and DMTMM is one reagent used for that reaction. The mechanism of DMTMM coupling is similar to other common amide coupling reactions involving activated carboxylic acids. Its precursor, 2-chloro-4,6,-dimethoxy-1,3,5-triazine (CDMT), has also been used for amide coupling. DMTMM has also been used to synthesize other carboxylic functional groups such as esters and anhydrides. DMTMM is usually used in the chloride form but the tetrafluoroborate salt is also commercially available.

Fomesafen is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase (PPO) which is necessary for chlorophyll synthesis. Soybeans naturally have a high tolerance to fomesafen, via metabolic disposal by glutathione S-transferase. As a result, soy is the most common crop treated with fomesafen, followed by other beans and a few other crop types. It is not safe for maize/corn or other Poaceae.

Aclonifen is a diphenyl ether herbicide which has been used in agriculture since the 1980s. Its mode of action has been uncertain, with evidence suggesting it might interfere with carotenoid biosynthesis or inhibit the enzyme protoporphyrinogen oxidase (PPO). Both mechanisms could result in the observed whole-plant effect of bleaching and the compound includes chemical features that are known to result in PPO effects, as seen with acifluorfen, for example. In 2020, further research revealed that aclonifen has a different and novel mode of action, targeting solanesyl diphosphate synthase which would also cause bleaching.
















