
Phenoxy herbicides (or "phenoxies") are two families of chemicals that have been developed as commercially important herbicides, widely used in agriculture. They share the part structure of phenoxyacetic acid.

Phenoxy herbicides (or "phenoxies") are two families of chemicals that have been developed as commercially important herbicides, widely used in agriculture. They share the part structure of phenoxyacetic acid.
The first group to be discovered act by mimicking the auxin growth hormone indoleacetic acid (IAA). [1] When sprayed on broad-leaf plants they induce rapid, uncontrolled growth ("growing to death"). Thus when applied to monocotyledonous crops such as wheat or maize (corn), they selectively kill broad-leaf weeds, leaving the crops relatively unaffected.
Introduced in 1946, these herbicides were in widespread use in agriculture by the middle of the 1950s. The best known phenoxy herbicides are (4-chloro-2-methylphenoxy)acetic acid (MCPA), 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T). [2] Analogues of each of these three compounds, with an extra methyl group attached next to the carboxylic acid, were subsequently commercialised as mecoprop, dichlorprop and fenoprop. The addition of the methyl group creates a chiral centre in these molecules and biological activity is found only in the (2R)-isomer (illustrated for dichlorprop). [3]
Other members of this group include 4-(2,4-dichlorophenoxy)butyric acid (2,4-DB) and 4-(4-chloro-2-methylphenoxy)butyric acid (MCPB) which act as propesticides for 2,4-D and MCPA respectively: that is, they are converted in plants to these active ingredients. [4] All the auxin herbicides retain activity when applied as salts and esters since these are also capable of producing the parent acid in situ.

The use of herbicides in US agriculture is mapped by the US Geological Survey. As of 2019 [update] , 2,4-D was the most used of the auxins. 45,000,000 pounds (20,000,000 kg) were sprayed that year, [5] compared to 2,000,000 pounds (910,000 kg) of the next most heavily applied, MCPA. [6] The other auxin now used in comparable amounts to 2,4-D is dicamba, where the 2019 figure was 30,000,000 pounds (14,000,000 kg). [7] It is a benzoic acid rather than a phenoxyacetic acid whose use has grown rapidly since 2016 as crops genetically modified to be resistant to it have been cultivated. [8]
In the 1970s, agrochemical companies were working to develop new herbicides to be complementary to the auxins. The aim was to find materials which would selectively control grass weeds in broad-leaf crops such as cotton and soybean.

In 1973, Hoechst AG filed patents on a new class of compound, the aryloxphenoxypropionates, which showed such selectivity and led to the commercialisation of diclofop. Then the Japanese company Ishihara Sangyo Kaisha (ISK) found improved biological activity in an analogue, chlorazifop, which replaced the aryloxy portion of diclofop with a pyridine ring containing the same two chlorine substituents. This area of research became very competitive and within three weeks of one another in 1977 ISK, Dow Chemicals and Imperial Chemical Industries (ICI) all filed patents covering another group of analogues, with a trifluoromethyl (CF3) group in place of one of the chlorine atoms in the pyridine. Subsequently, ISK and ICI cross-licensed their intellectual property and first marketed fluazifop as its butyl ester in 1981 under the brand name Fusilade while Dow marketed haloxyfop as its methyl ester. [9] All these compounds have an additional oxygen-linked aromatic group in the para position of the phenyl ring with its OCH(CH3)COOH group and as a class are called "fops", referring to their common fenoxy-phenoxy [sic] feature. [10]
This group of herbicides acts by inhibiting plant acetyl-CoA carboxylase (ACCase), a completely different mechanism of action to that of the auxins. [11] [12] Their selectivity for grasses arises because they target the isoform of the enzyme present only in the plastids of these species, making them ineffective on broad-leaf weeds and other organisms including mammals. [13] When applied as an ester, metabolism in the target plant leads to the parent acid which is responsible for the herbicidal action. [9] [14] It is a coincidence that it is the (2R) stereoisomer which binds to plant ACCase, just as that isomer is responsible for the activity of dichlorprop as an auxin.
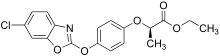

Salts and esters of this class of herbicide are active owing to their ability to metabolise to the corresponding parent acid. For example, fenoxaprop-P ethyl [15] was introduced by Bayer Crop Science and quizalofop-P ethyl by Nissan Chemical Corporation, both in 1989. [16] In 1990, Dow introduced cyhalofop-P butyl for the control of weeds in rice. [17] Fluazifop-P butyl [18] still has significant use in the USA. 200,000 pounds (91,000 kg) were applied in 2018 — almost exclusively in soyabean. [19] The "P" in the name of these materials refers to their use now as single enantiomers.
Cummins et al., 1999, 2009, and 2013 find that Alopecurus myocuroides 's mechanism of fenoxaprop-P-ethyl resistance reduces hydrogen peroxide concentrations at the application site, while the wild type responds with an increase. [20]

Herbicides, also commonly known as weed killers, are substances used to control undesired plants, also known as weeds. Selective herbicides control specific weed species while leaving the desired crop relatively unharmed, while non-selective herbicides kill plants indiscriminately. The combined effects of herbicides, nitrogen fertilizer, and improved cultivars has increased yields of major crops by 3x to 6x from 1900 to 2000.

Operation Ranch Hand was a U.S. military operation during the Vietnam War, lasting from 1962 until 1971. Largely inspired by the British use of chemicals 2,4,5-T and 2,4-D during the Malayan Emergency in the 1950s, it was part of the overall herbicidal warfare program during the war called "Operation Trail Dust". Ranch Hand involved spraying an estimated 19 million U.S. gallons (72,000 m3) of defoliants and herbicides over rural areas of South Vietnam in an attempt to deprive the Viet Cong of food and vegetation cover. Areas of Laos and Cambodia were also sprayed to a lesser extent. According to the Vietnamese government, the chemicals caused 400,000 deaths. The United States government has described these figures as unreliable.

Auxins are a class of plant hormones with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essential for plant body development. The Dutch biologist Frits Warmolt Went first described auxins and their role in plant growth in the 1920s. Kenneth V. Thimann became the first to isolate one of these phytohormones and to determine its chemical structure as indole-3-acetic acid (IAA). Went and Thimann co-authored a book on plant hormones, Phytohormones, in 1937.

A defoliant is any herbicidal chemical sprayed or dusted on plants to cause their leaves to fall off. Defoliants are widely used for the selective removal of weeds in managing croplands and lawns. Worldwide use of defoliants, along with the development of other herbicides and pesticides, allowed for the Green Revolution, an increase in agricultural production in mid-20th century. Defoliants have also been used in warfare as a means to deprive an enemy of food crops and/or hiding cover, most notably by the United Kingdom during the Malayan Emergency and the United States in the Vietnam War. Defoliants were also used by Indonesian forces in various internal security operations.

MCPA is a widely used phenoxy herbicide introduced in 1945. It selectively controls broad-leaf weeds in pasture and cereal crops. The mode of action of MCPA is as an auxin, which are growth hormones that naturally exist in plants.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

2,4,5-Trichlorophenoxyacetic acid, a synthetic auxin, is a chlorophenoxy acetic acid herbicide used to defoliate broad-leafed plants. It was developed in the late 1940s, synthesized by reaction of 2,4,5-Trichlorophenol and chloroacetic acid. It was widely used in the agricultural industry until being phased out, starting in the late 1970s due to toxicity concerns. Agent Orange, a defoliant used by the British in the Malayan Emergency and the U.S. in the Vietnam War, was equal parts 2,4,5-T and 2,4-D. 2,4,5-T itself is toxic with a NOAEL of 3 mg/kg/day and a LOAEL of 10 mg/kg/day. Agent Pink contained 100% 2,4,5-T. Additionally, the manufacturing process for 2,4,5-T contaminates this chemical with trace amounts of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). TCDD is a carcinogenic persistent organic pollutant with long-term effects on the environment. With proper temperature control during production of 2,4,5-T, TCDD levels can be held to about .005 ppm. Before the TCDD risk was well understood, early production facilities lacked proper temperature controls and individual batches tested later were found to have as much as 60 ppm of TCDD.
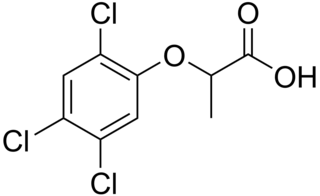
Fenoprop, also called 2,4,5-TP, is the organic compound 2-(2,4,5-trichlorophenoxy)propionic acid. It is a phenoxy herbicide and a plant growth regulator, an analog of 2,4,5-T in which the latter's acetic acid sidechain is replaced with a propionate group (with an extra CH3). The addition of this extra methyl group creates a chiral centre in the molecule and useful biological activity is found only in the (2R)-isomer. The compound's mechanism of action is to mimic the auxin growth hormone indoleacetic acid (IAA). When sprayed on plants it induces rapid, uncontrolled growth. As with 2,4,5-T, fenoprop is toxic to shrubs and trees.
Dicamba is a selective systemic herbicide first registered in 1967. Brand names for formulations of this herbicide include Dianat, Banvel, Diablo, Oracle and Vanquish. This chemical compound is a chlorinated derivative of o-anisic acid. It has been described as a "widely used, low-cost, environmentally friendly herbicide that does not persist in soils and shows little or no toxicity to wildlife and humans."

Mecoprop is a common general use herbicide found in many household weed killers and "weed-and-feed" type lawn fertilizers. It is primarily used to control broadleaf weeds. It is often used in combination with other chemically related herbicides such as 2,4-D, dicamba, and MCPA, which mimic the plant hormone IAA (auxin) and kill most broadleaf weeds by causing uncontrolled growth.
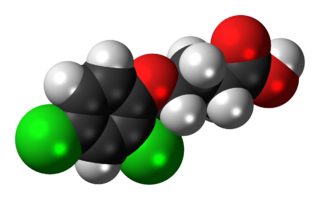
2,4-DB or 4-(2,4-dichlorophenoxy)butyric acid is a selective systemic phenoxy herbicide used to control many annual and perennial broad-leaf weeds in alfalfa, peanuts, soybeans, and other crops. Its active metabolite, 2,4-D, inhibits growth at the tips of stems and roots. It is classified in toxicity class III. It shows some evidence of toxicity to dogs and cats, such as changes in body weight and reduced numbers of offspring, when fed 25-80 milligrams per kilogram of body weight for prolonged periods. Tests of carcinogenicity in this range yielded differing results. It is moderately toxic to fish.
This is an index of articles relating to pesticides.

Saflufenacil is the ISO common name for an organic compound of the pyrimidinedione chemical class used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase to control broadleaf weeds in crops including soybeans and corn.
Chlorophenoxy herbicides are a subclass of phenoxy herbicides which includes: MCPA, 2,4-D, 2,4,5-T and mecoprop. Large amounts have been produced since the 1950s for agriculture. Acute toxic effects after oral consumption are varied and may include: vomiting, abdominal pain, diarrhoea, gastrointestinal haemorrhage acutely followed by coma, hypertonia, hyperreflexia, ataxia, nystagmus, miosis, hallucinations and convulsions. Treatment with urinary alkalinization may be helpful but evidence to support this practice is limited.

2,4-Dichlorophenoxyacetic acid is an organic compound with the chemical formula Cl2C6H3OCH2CO2H. It is usually referred to by its ISO common name 2,4-D. It is a systemic herbicide that kills most broadleaf weeds by causing uncontrolled growth, but most grasses such as cereals, lawn turf, and grassland are relatively unaffected.
The Enlist Weed Control System is an agricultural system that includes seeds for genetically modified crops that are resistant to Enlist and the Enlist herbicide; spraying the herbicide will kill weeds but not the resulting crop. The system was developed by Dow AgroSciences, part of Dow Chemical Company. In October 2014 the system was registered for restricted use in Illinois, Indiana, Iowa, Ohio, South Dakota and Wisconsin by the US Environmental Protection Agency. In 2013, the system was approved by Canada for the same uses.

Fluazifop is the common name used by the ISO for an organic compound that is used as a selective herbicide. The active ingredient is the 2R enantiomer at its chiral centre and this material is known as fluazifop-P when used in that form. More commonly, it is sold as its butyl ester, fluazifop-P butyl with the brand name Fusilade.

Butafenacil is the ISO common name for an organic compound of the pyrimidinedione chemical class used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase to control broadleaf and some grass weeds in crops including cereals and canola.

Aclonifen is a diphenyl ether herbicide which has been used in agriculture since the 1980s. Its mode of action has been uncertain, with evidence suggesting it might interfere with carotenoid biosynthesis or inhibit the enzyme protoporphyrinogen oxidase (PPO). Both mechanisms could result in the observed whole-plant effect of bleaching and the compound includes chemical features that are known to result in PPO effects, as seen with acifluorfen, for example. In 2020, further research revealed that aclonifen has a different and novel mode of action, targeting solanesyl diphosphate synthase which would also cause bleaching.