Herbicides, also commonly known as weed killers, are substances used to control undesired plants, also known as weeds. Selective herbicides control specific weed species while leaving the desired crop relatively unharmed, while non-selective herbicides kill plants indiscriminately. The combined effects of herbicides, nitrogen fertilizer, and improved cultivars has increased yields of major crops by 3x to 6x from 1900 to 2000.

Diquat is the ISO common name for an organic dication that, as a salt with counterions such as bromide or chloride is used as a contact herbicide that produces desiccation and defoliation. Diquat is no longer approved for use in the European Union, although its registration in many other countries including the USA is still valid.

Phenoxy herbicides are two families of chemicals that have been developed as commercially important herbicides, widely used in agriculture. They share the part structure of phenoxyacetic acid.

Glufosinate is a naturally occurring broad-spectrum herbicide produced by several species of Streptomyces soil bacteria. Glufosinate is a non-selective, contact herbicide, with some systemic action. Plants may also metabolize bialaphos and phosalacine, other naturally occurring herbicides, directly into glufosinate. The compound irreversibly inhibits glutamine synthetase, an enzyme necessary for the production of glutamine and for ammonia detoxification, giving it antibacterial, antifungal and herbicidal properties. Application of glufosinate to plants leads to reduced glutamine and elevated ammonia levels in tissues, halting photosynthesis and resulting in plant death.

4-Hydroxyphenylpyruvate dioxygenase (HPPD), also known as α-ketoisocaproate dioxygenase, is an Fe(II)-containing non-heme oxygenase that catalyzes the second reaction in the catabolism of tyrosine - the conversion of 4-hydroxyphenylpyruvate into homogentisate. HPPD also catalyzes the conversion of phenylpyruvate to 2-hydroxyphenylacetate and the conversion of α-ketoisocaproate to β-hydroxy β-methylbutyrate. HPPD is an enzyme that is found in nearly all aerobic forms of life.

Nitisinone, sold under the brand name Orfadin among others, is a medication used to slow the effects of hereditary tyrosinemia type 1 (HT-1).

Azoxystrobin is a broad spectrum systemic fungicide widely used in agriculture to protect crops from fungal diseases. It was first marketed in 1996 using the brand name Amistar and by 1999 it had been registered in 48 countries on more than 50 crops. In the year 2000 it was announced that it had been granted UK Millennium product status.
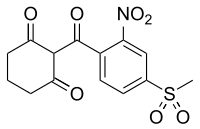
Sulfentrazone is the ISO common name for an organic compound used as a broad-spectrum herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase. It was first marketed in the US in 1997 by FMC Corporation with the brand name Authority.

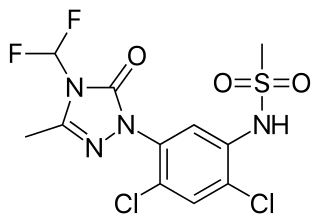
Saflufenacil is the ISO common name for an organic compound of the pyrimidinedione chemical class used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase to control broadleaf weeds in crops including soybeans and corn.
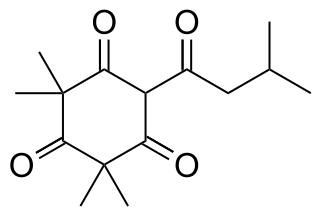
Leptospermone is a chemical compound produced by some members of the myrtle family (Myrtaceae), such as Callistemon citrinus, a shrub native to Australia, and Leptospermum scoparium (Manuka), a New Zealand tree from which it gets its name. Modification of this allelopathic chemical to produce mesotrione led to the commercialization of derivative compounds as HPPD inhibitor herbicides.

2,4-Dichlorophenoxyacetic acid is an organic compound with the chemical formula Cl2C6H3OCH2CO2H. It is usually referred to by its ISO common name 2,4-D. It is a systemic herbicide that kills most broadleaf weeds by causing uncontrolled growth, but most grasses such as cereals, lawn turf, and grassland are relatively unaffected.
4-Hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors are a class of herbicides that prevent growth in plants by blocking 4-Hydroxyphenylpyruvate dioxygenase, an enzyme in plants that breaks down the amino acid tyrosine into molecules that are then used by plants to create other molecules that plants need. This process of breakdown, or catabolism, and making new molecules from the results, or biosynthesis, is something all living things do. HPPD inhibitors were first brought to market in 1980, although their mechanism of action was not understood until the late 1990s. They were originally used primarily in Japan in rice production, but since the late 1990s have been used in Europe and North America for corn, soybeans, and cereals, and since the 2000s have become more important as weeds have become resistant to glyphosate and other herbicides. Genetically modified crops are under development that include resistance to HPPD inhibitors. There is a pharmaceutical drug on the market, nitisinone, that was originally under development as an herbicide as a member of this class, and is used to treat an orphan disease, type I tyrosinemia.
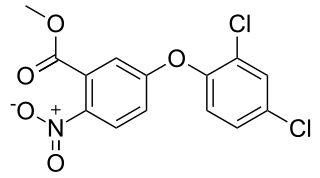
Bifenox is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase which is necessary for chlorophyll synthesis.
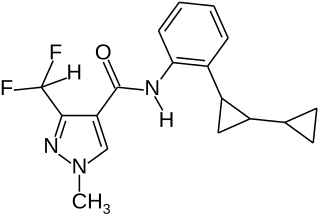
Sedaxane is a broad spectrum fungicide used as a seed treatment in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2011 using their brand name Vibrance. The compound is an amide which combines a pyrazole acid with an aryl amine to give an inhibitor of succinate dehydrogenase.

Fomesafen is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase (PPO) which is necessary for chlorophyll synthesis. Soybeans naturally have a high tolerance to fomesafen, via metabolic disposal by glutathione S-transferase. As a result, soy is the most common crop treated with fomesafen, followed by other beans and a few other crop types. It is not safe for maize/corn or other Poaceae.

Fluazifop is the common name used by the ISO for an organic compound that is used as a selective herbicide. The active ingredient is the 2R enantiomer at its chiral centre and this material is known as fluazifop-P when used in that form. More commonly, it is sold as its butyl ester, fluazifop-P butyl with the brand name Fusilade.

Indaziflam is a preemergent herbicide especially for grass control in tree and bush crops.

Butafenacil is the ISO common name for an organic compound of the pyrimidinedione chemical class used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase to control broadleaf and some grass weeds in crops including cereals and canola.

Pydiflumetofen is a broad spectrum fungicide used in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2016 using their brand name Miravis. The compound is an amide which combines a pyrazole acid with a substituted phenethylamine to give an inhibitor of succinate dehydrogenase, an enzyme that inhibits cellular respiration in almost all living organisms.
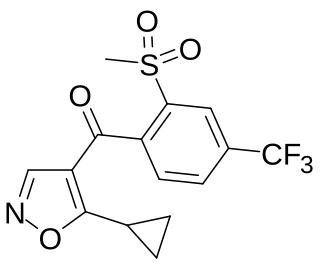
Isoxaflutole is a selective herbicide used mainly in maize crops. It inhibits the enzyme 4-hydroxyphenylpyruvate dioxygenase (HPPD) and is sold under brand names including Balance and Merlin. It was first marketed by Rhône-Poulenc in 1996.