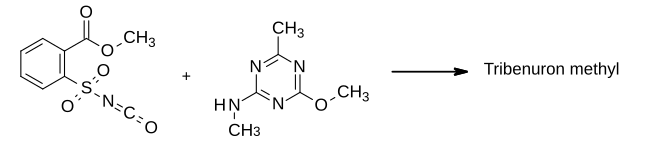
Herbicides, also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds. Selective herbicides control specific weed species, while leaving the desired crop relatively unharmed, while non-selective herbicides can be used to clear waste ground, industrial and construction sites, railways and railway embankments as they kill all plant material with which they come into contact. Apart from selective/non-selective, other important distinctions include persistence, means of uptake, and mechanism of action. Historically, products such as common salt and other metal salts were used as herbicides, however these have gradually fallen out of favor and in some countries a number of these are banned due to their persistence in soil, and toxicity and groundwater contamination concerns. Herbicides have also been used in warfare and conflict.

Pesticide resistance describes the decreased susceptibility of a pest population to a pesticide that was previously effective at controlling the pest. Pest species evolve pesticide resistance via natural selection: the most resistant specimens survive and pass on their acquired heritable changes traits to their offspring. If a pest has resistance then the pesticide lacks efficacy – efficacy and resistance are inversely related.

Weed control is a type of pest control, which attempts to stop or reduce growth of weeds, especially noxious weeds, with the aim of reducing their competition with desired flora and fauna including domesticated plants and livestock, and in natural settings preventing non native species competing with native species.

MCPA is a powerful, selective, widely used phenoxy herbicide. The pure compound is a brown-colored powder. MCPA has been extensively used in agriculture to control broad-leaf weeds as a growth regulator primarily in pasture and cereal crops field since 1945. The mode of action of MCPA is as an auxin, which are growth hormones that naturally exist in plants. Overdose application of MCPA acts as an herbicide and results in abnormal growth.
Chlortoluron or chlorotoluron are the common names for an organic compound of the phenylurea class of herbicides used to control broadleaf and annual grass weeds in cereal crops.

Phenoxy herbicides are two families of chemicals that have been developed as commercially important herbicides, widely used in agriculture. They share the part structure of phenoxyacetic acid.

DCMU is an algicide and herbicide of the arylurea class that inhibits photosynthesis. It was introduced by Bayer in 1954 under the trade name of Diuron.
A mode of action (MoA) describes a functional or anatomical change, resulting from the exposure of a living organism to a substance. In comparison, a mechanism of action (MOA) describes such changes at the molecular level.

Pendimethalin is an herbicide of the dinitroaniline class used in premergence and postemergence applications to control annual grasses and certain broadleaf weeds. It inhibits cell division and cell elongation. Pendimethalin is listed in the K1-group according to the Herbicide Resistance Action Committee (HRAC) classification and is approved in Europe, North America, South America, Africa, Asia and Oceania for different crops including cereals, corn, soybeans, rice, potato, legumes, fruits, vegetables, nuts as well as lawns and ornamental plants.

The acetolactate synthase (ALS) enzyme is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids.

Orobanche aegyptiaca, the Egyptian broomrape, is a plant which is an obligate holoparasite from the family Orobanchaceae with a complex lifecycle. This parasite is most common in the Middle East and has a wide host range including many economically important crops.
Mesotrione is the ISO common name for an organic compound that is used as a selective herbicide, especially in maize. A synthetic inspired by the natural substance leptospermone, it inhibits the enzyme 4-hydroxyphenylpyruvate dioxygenase (HPPD) and is sold under brand names including Callisto and Tenacity. It was first marketed by Syngenta in 2001.
4-Hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors are a class of herbicides that prevent growth in plants by blocking 4-Hydroxyphenylpyruvate dioxygenase, an enzyme in plants that breaks down the amino acid tyrosine into molecules that are then used by plants to create other molecules that plants need. This process of breakdown, or catabolism, and making new molecules from the results, or biosynthesis, is something all living things do. HPPD inhibitors were first brought to market in 1980, although their mechanism of action was not understood until the late 1990s. They were originally used primarily in Japan in rice production, but since the late 1990s have been used in Europe and North America for corn, soybeans, and cereals, and since the 2000s have become more important as weeds have become resistant to glyphosate and other herbicides. Genetically modified crops are under development that include resistance to HPPD inhibitors. There is a pharmaceutical drug on the market, nitisinone, that was originally under development as an herbicide as a member of this class, and is used to treat an orphan disease, type I tyrosinemia.

Imazaquin is an imidazolinone herbicide, so named because it contains an imidazolinone core. This organic compound is used to control a broad spectrum of weed species. It is a colorless or white solid, although commercial samples can appear brown or tan.
Flazasulfuron is an organic compound that is used as a herbicide. It is classified as a sulfonylurea, because it contains that functional group. The mode of action of flazasulfuron is the inhibition of the enzyme acetolactate synthase (ALS), which results in the inhibition of amino acid synthesis, cell division and ultimately plant growth. Flazasulfuron can be used on both pre-emergent weeds and post-emergent weeds. Growth ceases within hours of the application of the compound. Symptoms include leaf discolouration, desiccation, necrosis and ultimately plant death within 20 – 25 days of application. It is a white, water-soluble solid.

Fomesafen is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase which is necessary for chlorophyll synthesis. Soybeans naturally have a high tolerance to fomesafen, via metabolic disposal by glutathione S-transferase. As a result, soy is the most common crop treated with fomesafen, followed by other beans and a few other crop types. It is not safe for maize/corn or other Poaceae.

Fluazifop is the ISO common name for an organic compound that is used as a selective herbicide. The active ingredient is the 2R enantiomer at its chiral centre and this material is known as fluazifop-P when used in that form. More commonly, it is sold as its butyl ester, fluazifop-P butyl with the brand name Fusilade.

Indaziflam is a preemergent herbicide especially for grass control in tree and bush crops.

Aclonifen is a diphenyl ether herbicide which has been used in agriculture since the 1980s. Its mode of action has been uncertain, with evidence suggesting it might interfere with carotenoid biosynthesis or inhibit the enzyme protoporphyrinogen oxidase (PPO). Both mechanisms could result in the observed whole-plant effect of bleaching and the compound includes chemical features that are known to result in PPO effects, as seen with acifluorfen, for example. In 2020, further research revealed that aclonifen has a different and novel mode of action, targeting solanesyl diphosphate synthase which would also cause bleaching.

Chlorsulfuron is an ALS inhibitor herbicide, and is a sulfonylurea compound. It was discovered by George Levitt in February 1976 while working at DuPont, which was the patent assignee.