The heart is a muscular organ in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to the lungs. In humans, the heart is approximately the size of a closed fist and is located between the lungs, in the middle compartment of the chest, called the mediastinum.

A heart valve is a biological one-way valve that allows blood to flow in one direction through the chambers of the heart. Four valves are usually present in a mammalian heart and together they determine the pathway of blood flow through the heart. A heart valve opens or closes according to differential blood pressure on each side.

Systole is the part of the cardiac cycle during which some chambers of the heart contract after refilling with blood.

A ventricle is one of two large chambers located toward the bottom of the heart that collect and expel blood towards the peripheral beds within the body and lungs. The blood pumped by a ventricle is supplied by an atrium, an adjacent chamber in the upper heart that is smaller than a ventricle. Interventricular means between the ventricles, while intraventricular means within one ventricle.

The cardiac conduction system transmits the signals generated by the sinoatrial node – the heart's pacemaker, to cause the heart muscle to contract, and pump blood through the body's circulatory system. The pacemaking signal travels through the right atrium to the atrioventricular node, along the bundle of His, and through the bundle branches to Purkinje fibers in the walls of the ventricles. The Purkinje fibers transmit the signals more rapidly to stimulate contraction of the ventricles.

Atrial septal defect (ASD) is a congenital heart defect in which blood flows between the atria of the heart. Some flow is a normal condition both pre-birth and immediately post-birth via the foramen ovale; however, when this does not naturally close after birth it is referred to as a patent (open) foramen ovale (PFO). It is common in patients with a congenital atrial septal aneurysm (ASA).

The ostium primum atrial septal defect is a defect in the atrial septum at the level of the tricuspid and mitral valves. This is sometimes known as an endocardial cushion defect because it often involves the endocardial cushion, which is the portion of the heart where the atrial septum meets the ventricular septum and the mitral valve meets the tricuspid valve.

A congenital heart defect (CHD), also known as a congenital heart anomaly, congenital cardiovascular malformation, and congenital heart disease, is a defect in the structure of the heart or great vessels that is present at birth. A congenital heart defect is classed as a cardiovascular disease. Signs and symptoms depend on the specific type of defect. Symptoms can vary from none to life-threatening. When present, symptoms are variable and may include rapid breathing, bluish skin (cyanosis), poor weight gain, and feeling tired. CHD does not cause chest pain. Most congenital heart defects are not associated with other diseases. A complication of CHD is heart failure.

The atrium is one of the two upper chambers in the heart that receives blood from the circulatory system. The blood in the atria is pumped into the heart ventricles through the atrioventricular mitral and tricuspid heart valves.

The cardiac cycle is the performance of the human heart from the beginning of one heartbeat to the beginning of the next. It consists of two periods: one during which the heart muscle relaxes and refills with blood, called diastole, following a period of robust contraction and pumping of blood, called systole. After emptying, the heart relaxes and expands to receive another influx of blood returning from the lungs and other systems of the body, before again contracting to pump blood to the lungs and those systems.

Atrioventricular septal defect (AVSD) or atrioventricular canal defect (AVCD), also known as "common atrioventricular canal" or "endocardial cushion defect" (ECD), is characterized by a deficiency of the atrioventricular septum of the heart that creates connections between all four of its chambers. It is a very specific combination of 3 defects:

The coronary sinus is the largest vein of the heart. It drains over half of the deoxygenated blood from the heart muscle into the right atrium. It begins on the backside of the heart, in between the left atrium, and left ventricle; it begins at the junction of the great cardiac vein, and oblique vein of the left atrium. It receives multiple tributaries. It passes across the backside of the heart along a groove between left atrium and left ventricle, then drains into the right atrium at the orifice of the coronary sinus.
Cor triatriatum is a congenital heart defect where the left atrium or right atrium is subdivided by a thin membrane, resulting in three atrial chambers.

The sinus venosus is a large quadrangular cavity which precedes the atrium on the venous side of the chordate heart.
The proper development of the atrioventricular canal into its prospective components to create a clear division between the four compartments of the heart and ensure proper blood movement through the heart, are essential for proper heart function. When this process does not happen correctly, a child will develop atrioventricular canal defect which occurs in 2 out of every 10,000 births. It also has a correlation with Down syndrome because 20% of children with Down syndrome have atrioventricular canal disease as well. This is a very serious condition and surgery is necessary within the first six months of life for a child. Half of the children who are untreated with this condition die during their first year due to heart failure or pneumonia.

The valve of the inferior vena cava is a venous valve that lies at the junction of the inferior vena cava and right atrium.

The truncus arteriosus is a structure that is present during embryonic development. It is an arterial trunk that originates from both ventricles of the heart that later divides into the aorta and the pulmonary trunk.
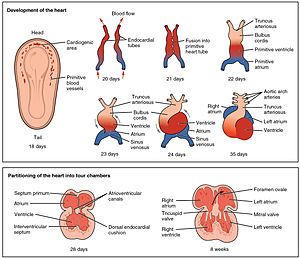
The heart is the first functional organ in a vertebrate embryo. There are 5 stages to heart development.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.
The heart is a muscular organ situated in the mediastinum. It consists of four chambers, four valves, two main arteries, and the conduction system. The left and right sides of the heart have different functions: the right side receives de-oxygenated blood through the superior and inferior venae cavae and pumps blood to the lungs through the pulmonary artery, and the left side receives saturated blood from the lungs.