Tumor necrosis factor is a cytokine and member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain. It is the first cytokine to be described as an adipokine as secreted by adipose tissue.

Lipolysis is the metabolic pathway through which lipid triglycerides are hydrolyzed into a glycerol and free fatty acids. It is used to mobilize stored energy during fasting or exercise, and usually occurs in fat adipocytes. The most important regulatory hormone in lipolysis is insulin; lipolysis can only occur when insulin action falls to low levels, as occurs during fasting. Other hormones that affect lipolysis include leptin, glucagon, epinephrine, norepinephrine, growth hormone, atrial natriuretic peptide, brain natriuretic peptide, and cortisol.

A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass. These cells are involved in:

Fibrosis, also known as fibrotic scarring, is a pathological wound healing in which connective tissue replaces normal parenchymal tissue to the extent that it goes unchecked, leading to considerable tissue remodelling and the formation of permanent scar tissue.

Fatty liver disease (FLD), also known as hepatic steatosis and steatotic liver disease (SLD), is a condition where excess fat builds up in the liver. Often there are no or few symptoms. Occasionally there may be tiredness or pain in the upper right side of the abdomen. Complications may include cirrhosis, liver cancer, and esophageal varices.

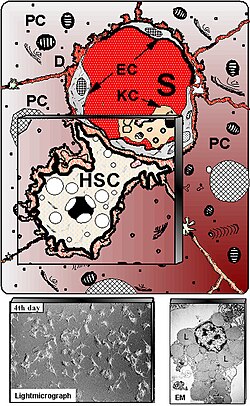
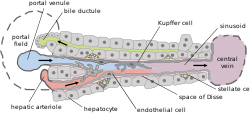
Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells comprise the largest population of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis. They form part of the mononuclear phagocyte system.
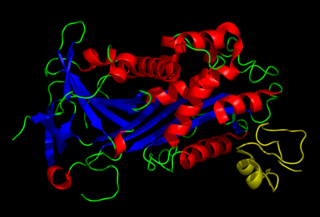
Plasminogen activator inhibitor-1 (PAI-1) also known as endothelial plasminogen activator inhibitor is a protein that in humans is encoded by the SERPINE1 gene. Elevated PAI-1 is a risk factor for thrombosis and atherosclerosis.

Hypervitaminosis A refers to the toxic effects of ingesting too much preformed vitamin A. Symptoms arise as a result of altered bone metabolism and altered metabolism of other fat-soluble vitamins. Hypervitaminosis A is believed to have occurred in early humans, and the problem has persisted throughout human history. Toxicity results from ingesting too much preformed vitamin A from foods, supplements, or prescription medications and can be prevented by ingesting no more than the recommended daily amount.

A myofibroblast is a cell phenotype that was first described as being in a state between a fibroblast and a smooth muscle cell.

Thy-1 or CD90 is a 25–37 kDa heavily N-glycosylated, glycophosphatidylinositol (GPI) anchored conserved cell surface protein with a single V-like immunoglobulin domain, originally discovered as a thymocyte antigen. Thy-1 can be used as a marker for a variety of stem cells and for the axonal processes of mature neurons. Structural study of Thy-1 led to the foundation of the Immunoglobulin superfamily, of which it is the smallest member, and led to some of the initial biochemical description and characterization of a vertebrate GPI anchor and also the first demonstration of tissue specific differential glycosylation.

The perisinusoidal space is a location in the liver between a hepatocyte and a sinusoid. It contains the blood plasma. Microvilli of hepatocytes extend into this space, allowing proteins and other plasma components from the sinusoids to be absorbed by the hepatocytes. Fenestration and discontinuity of the endothelium facilitates this transport. This space may be obliterated in liver disease, leading to decreased uptake by hepatocytes of nutrients and wastes such as bilirubin.

A liver sinusoid is a type of capillary known as a sinusoidal capillary, discontinuous capillary or sinusoid, that is similar to a fenestrated capillary, having discontinuous endothelium that serves as a location for mixing of the oxygen-rich blood from the hepatic artery and the nutrient-rich blood from the portal vein.

Cytoglobin is the protein product of CYGB, a human and mammalian gene.
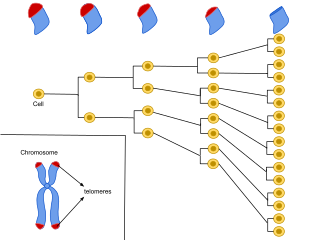
Cellular senescence is a phenomenon characterized by the cessation of cell division. In their experiments during the early 1960s, Leonard Hayflick and Paul Moorhead found that normal human fetal fibroblasts in culture reach a maximum of approximately 50 cell population doublings before becoming senescent. This process is known as "replicative senescence", or the Hayflick limit. Hayflick's discovery of mortal cells paved the path for the discovery and understanding of cellular aging molecular pathways. Cellular senescence can be initiated by a wide variety of stress inducing factors. These stress factors include both environmental and internal damaging events, abnormal cellular growth, oxidative stress, autophagy factors, among many other things.

The liver is a major metabolic organ only found in vertebrate animals, which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it is located in the right upper quadrant of the abdomen, below the diaphragm and mostly shielded by the lower right rib cage. Its other metabolic roles include carbohydrate metabolism, the production of hormones, conversion and storage of nutrients such as glucose and glycogen, and the decomposition of red blood cells.

Cirrhosis, also known as liver cirrhosis or hepatic cirrhosis, and end-stage liver disease, is the impaired liver function caused by the formation of scar tissue known as fibrosis due to damage caused by liver disease. Damage to the liver leads to repair of liver tissue and subsequent formation of scar tissue. Over time, scar tissue can replace normal functioning tissue, leading to the impaired liver function of cirrhosis. The disease typically develops slowly over months or years. Early symptoms may include tiredness, weakness, loss of appetite, unexplained weight loss, nausea and vomiting, and discomfort in the right upper quadrant of the abdomen. As the disease worsens, symptoms may include itchiness, swelling in the lower legs, fluid build-up in the abdomen, jaundice, bruising easily, and the development of spider-like blood vessels in the skin. The fluid build-up in the abdomen may develop into spontaneous infections. More serious complications include hepatic encephalopathy, bleeding from dilated veins in the esophagus, stomach, or intestines, and liver cancer. Stages of cirrhosis include compensated cirrhosis and decompensated cirrhosis.
Pancreatic stellate cells (PaSCs) are classified as myofibroblast-like cells that are located in exocrine regions of the pancreas. PaSCs are mediated by paracrine and autocrine stimuli and share similarities with the hepatic stellate cell. Pancreatic stellate cell activation and expression of matrix molecules constitute the complex process that induces pancreatic fibrosis. Synthesis, deposition, maturation and remodelling of the fibrous connective tissue can be protective, however when persistent it impedes regular pancreatic function.
Liver cytology is the branch of cytology that studies the liver cells and its functions. The liver is a vital organ, in charge of almost all the body’s metabolism. Main liver cells are hepatocytes, Kupffer cells, and hepatic stellate cells; each one with a specific function.
Liver regeneration is the process by which the liver is able to replace damaged or lost liver tissue. The liver is the only visceral organ with the capacity to regenerate. The liver can regenerate after partial hepatectomy or injury due to hepatotoxic agents such as certain medications, toxins, or chemicals. Only 51% of the original liver mass is required for the organ to regenerate back to full size. The phenomenon of liver regeneration is seen in all vertebrates, from humans to fish. The liver manages to restore any lost mass and adjust its size to that of the organism, while at the same time providing full support for body homeostasis during the entire regenerative process. The process of regeneration in mammals is mainly compensatory growth or hyperplasia because while the lost mass of the liver is replaced, it does not regain its original shape. During compensatory hyperplasia, the remaining liver tissue becomes larger so that the organ can continue to function. In lower species such as fish, the liver can regain both its original size and mass.
Liver sinusoidal endothelial cells (LSECs) form the lining of the smallest blood vessels in the liver, also called the hepatic sinusoids. LSECs are highly specialized endothelial cells with characteristic morphology and function. They constitute an important part of the reticuloendothelial system (RES).