
A scar is an area of fibrous tissue that replaces normal skin after an injury. Scars result from the biological process of wound repair in the skin, as well as in other organs, and tissues of the body. Thus, scarring is a natural part of the healing process. With the exception of very minor lesions, every wound results in some degree of scarring. An exception to this are animals with complete regeneration, which regrow tissue without scar formation.

In biology, the extracellular matrix (ECM), also called intercellular matrix (ICM), is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.

With physical trauma or disease suffered by an organism, healing involves the repairing of damaged tissue(s), organs and the biological system as a whole and resumption of (normal) functioning. Medicine includes the process by which the cells in the body regenerate and repair to reduce the size of a damaged or necrotic area and replace it with new living tissue. The replacement can happen in two ways: by regeneration in which the necrotic cells are replaced by new cells that form "like" tissue as was originally there; or by repair in which injured tissue is replaced with scar tissue. Most organs will heal using a mixture of both mechanisms.

Smooth(soft) muscle is an involuntary non-striated muscle, so-called because it has no sarcomeres and therefore no striations. It is divided into two subgroups, single-unit and multiunit smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium.
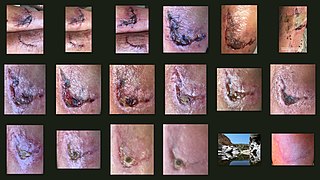
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.
Mesangial cells are specialised cells in the kidney that make up the mesangium of the glomerulus. Together with the mesangial matrix, they form the vascular pole of the renal corpuscle. The mesangial cell population accounts for approximately 30-40% of the total cells in the glomerulus. Mesangial cells can be categorized as either extraglomerular mesangial cells or intraglomerular mesangial cells, based on their relative location to the glomerulus. The extraglomerular mesangial cells are found between the afferent and efferent arterioles towards the vascular pole of the glomerulus. The extraglomerular mesangial cells are adjacent to the intraglomerular mesangial cells that are located inside the glomerulus and in between the capillaries. The primary function of mesangial cells is to remove trapped residues and aggregated protein from the basement membrane thus keeping the filter free of debris. The contractile properties of mesangial cells have been shown to be insignificant in changing the filtration pressure of the glomerulus.

Fibrosis, also known as fibrotic scarring, is a pathological wound healing in which connective tissue replaces normal parenchymal tissue to the extent that it goes unchecked, leading to considerable tissue remodelling and the formation of permanent scar tissue.

Platelet-derived growth factor (PDGF) is one among numerous growth factors that regulate cell growth and division. In particular, PDGF plays a significant role in blood vessel formation, the growth of blood vessels from already-existing blood vessel tissue, mitogenesis, i.e. proliferation, of mesenchymal cells such as fibroblasts, osteoblasts, tenocytes, vascular smooth muscle cells and mesenchymal stem cells as well as chemotaxis, the directed migration, of mesenchymal cells. Platelet-derived growth factor is a dimeric glycoprotein that can be composed of two A subunits (PDGF-AA), two B subunits (PDGF-BB), or one of each (PDGF-AB).
Haptotaxis is the directional motility or outgrowth of cells, e.g. in the case of axonal outgrowth, usually up a gradient of cellular adhesion sites or substrate-bound chemoattractants. These gradients are naturally present in the extracellular matrix (ECM) of the body during processes such as angiogenesis or artificially present in biomaterials where gradients are established by altering the concentration of adhesion sites on a polymer substrate.
Durotaxis is a form of cell migration in which cells are guided by rigidity gradients, which arise from differential structural properties of the extracellular matrix (ECM). Most normal cells migrate up rigidity gradients.
A fibrocyte is an inactive mesenchymal cell, that is, a cell showing minimal cytoplasm, limited amounts of rough endoplasmic reticulum and lacks biochemical evidence of protein synthesis.

Hepatic stellate cells (HSC), also known as perisinusoidal cells or Ito cells, are pericytes found in the perisinusoidal space of the liver, also known as the space of Disse. The stellate cell is the major cell type involved in liver fibrosis, which is the formation of scar tissue in response to liver damage, in addition these cells store and concentrate vitamin A.

ACTA2 is an actin protein with several aliases including alpha-actin, alpha-actin-2,aortic smooth muscle or alpha smooth muscle actin. Actins are a family of globular multi-functional proteins that form microfilaments. ACTA2 is one of 6 different actin isoforms and is involved in the contractile apparatus of smooth muscle. ACTA2 is extremely highly conserved and found in nearly all mammals.

Integrin alpha-11 is a protein that, in humans, is encoded by the ITGA11 gene.

In medicine, desmoplasia is the growth of fibrous connective tissue. It is also called a desmoplastic reaction to emphasize that it is secondary to an insult. Desmoplasia may occur around a neoplasm, causing dense fibrosis around the tumor, or scar tissue (adhesions) within the abdomen after abdominal surgery.
Pancreatic stellate cells (PaSCs) are classified as myofibroblast-like cells that are located in exocrine regions of the pancreas. PaSCs are mediated by paracrine and autocrine stimuli and share similarities with the hepatic stellate cell. Pancreatic stellate cell activation and expression of matrix molecules constitute the complex process that induces pancreatic fibrosis. Synthesis, deposition, maturation and remodelling of the fibrous connective tissue can be protective, however when persistent it impedes regular pancreatic function.
Cenderitide is a natriuretic peptide developed by the Mayo Clinic as a potential treatment for heart failure. Cenderitide is created by the fusion of the 15 amino acid C-terminus of the snake venom dendroaspis natriuretic peptide (DNP) with the full C-type natriuretic peptide (CNP) structure. This peptide chimera is a dual activator of the natriuretic peptide receptors NPR-A and NPR-B and therefore exhibits the natriuretic and diuretic properties of DNP, as well as the antiproliferative and antifibrotic properties of CNP.
Dermal fibroblasts are cells within the dermis layer of skin which are responsible for generating connective tissue and allowing the skin to recover from injury. Using organelles, dermal fibroblasts generate and maintain the connective tissue which unites separate cell layers. Furthermore, these dermal fibroblasts produce the protein molecules including laminin and fibronectin which comprise the extracellular matrix. By creating the extracellular matrix between the dermis and epidermis, fibroblasts allow the epithelial cells of the epidermis to affix the matrix, thereby allowing the epidermal cells to effectively join together to form the top layer of the skin.
A cancer-associated fibroblast (CAF) is a cell type within the tumor microenvironment that promotes tumorigenic features by initiating the remodelling of the extracellular matrix or by secreting cytokines. CAFs are a complex and abundant cell type within the tumour microenvironment; the number cannot decrease, as they are unable to undergo apoptosis.

Invasion is the process by which cancer cells directly extend and penetrate into neighboring tissues in cancer. It is generally distinguished from metastasis, which is the spread of cancer cells through the circulatory system or the lymphatic system to more distant locations. Yet, lymphovascular invasion is generally the first step of metastasis.













