Related Research Articles

Allergies, also known as allergic diseases, are various conditions caused by hypersensitivity of the immune system to typically harmless substances in the environment. These diseases include hay fever, food allergies, atopic dermatitis, allergic asthma, and anaphylaxis. Symptoms may include red eyes, an itchy rash, sneezing, coughing, a runny nose, shortness of breath, or swelling. Note that food intolerances and food poisoning are separate conditions.

In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an "autoimmune disease". Prominent examples include celiac disease, diabetes mellitus type 1, Henoch–Schönlein purpura, systemic lupus erythematosus, Sjögren syndrome, eosinophilic granulomatosis with polyangiitis, Hashimoto's thyroiditis, Graves' disease, idiopathic thrombocytopenic purpura, Addison's disease, rheumatoid arthritis, ankylosing spondylitis, polymyositis, dermatomyositis, and multiple sclerosis. Autoimmune diseases are very often treated with steroids.
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
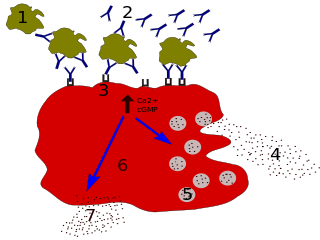
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.
Immunodeficiency, also known as immunocompromisation, is a state in which the immune system's ability to fight infectious diseases and cancer is compromised or entirely absent. Most cases are acquired ("secondary") due to extrinsic factors that affect the patient's immune system. Examples of these extrinsic factors include HIV infection and environmental factors, such as nutrition. Immunocompromisation may also be due to genetic diseases/flaws such as SCID.

Autoimmune polyendocrine syndromes (APSs), also called polyglandular autoimmune syndromes (PGASs) or polyendocrine autoimmune syndromes (PASs), are a heterogeneous group of rare diseases characterized by autoimmune activity against more than one endocrine organ, although non-endocrine organs can be affected. There are three types of APS, and there are a number of other diseases which involve endocrine autoimmunity.

FOXP3, also known as scurfin, is a protein involved in immune system responses. A member of the FOX protein family, FOXP3 appears to function as a master regulator of the regulatory pathway in the development and function of regulatory T cells. Regulatory T cells generally turn the immune response down. In cancer, an excess of regulatory T cell activity can prevent the immune system from destroying cancer cells. In autoimmune disease, a deficiency of regulatory T cell activity can allow other autoimmune cells to attack the body's own tissues.

The interleukin 4 is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13.
Common variable immunodeficiency (CVID) is an inborn immune disorder characterized by recurrent infections and low antibody levels, specifically in immunoglobulin (Ig) types IgG, IgM, and IgA. Symptoms generally include high susceptibility to pathogens, chronic lung disease, as well as inflammation and infection of the gastrointestinal tract.

Immunodysregulation polyendocrinopathy enteropathy X-linked syndrome is a rare autoimmune disease. It is one of the autoimmune polyendocrine syndromes. Most often, IPEX presents with autoimmune enteropathy, dermatitis (eczema), and autoimmune endocrinopathy, but other presentations exist.
Immune tolerance, also known as immunological tolerance or immunotolerance, refers to the immune system's state of unresponsiveness to substances or tissues that would otherwise trigger an immune response. It arises from prior exposure to a specific antigen and contrasts the immune system's conventional role in eliminating foreign antigens. Depending on the site of induction, tolerance is categorized as either central tolerance, occurring in the thymus and bone marrow, or peripheral tolerance, taking place in other tissues and lymph nodes. Although the mechanisms establishing central and peripheral tolerance differ, their outcomes are analogous, ensuring immune system modulation.

The autoimmune regulator (AIRE) is a protein that in humans is encoded by the AIRE gene. It is a 13kb gene on chromosome 21q22.3 that has 545 amino acids. AIRE is a transcription factor expressed in the medulla of the thymus. It is part of the mechanism which eliminates self-reactive T cells that would cause autoimmune disease. It exposes T cells to normal, healthy proteins from all parts of the body, and T cells that react to those proteins are destroyed.
An immune disorder is a dysfunction of the immune system. These disorders can be characterized in several different ways:

Autoimmune polyendocrine syndrome type 1 (APS-1), is a subtype of autoimmune polyendocrine syndrome. It causes the dysfunction of multiple endocrine glands due to autoimmunity. It is a genetic disorder, inherited in autosomal recessive fashion due to a defect in the AIRE gene , which is located on chromosome 21 and normally confers immune tolerance.
The effects of parasitic worms, or helminths, on the immune system is a recently emerging topic of study among immunologists and other biologists. Experiments have involved a wide range of parasites, diseases, and hosts. The effects on humans have been of special interest. The tendency of many parasitic worms to pacify the host's immune response allows them to mollify some diseases, while worsening others.

CD25 deficiency or interleukin 2 receptor alpha deficiency is an immunodeficiency disorder associated with mutations in the interleukin 2 receptor alpha (CD25) (IL2RA) gene. The mutations cause expression of a defective α chain or complete absence thereof, an essential part of high-affinity interleukin-2 (IL-2) receptors. The result is a syndrome described as IPEX-like or a SCID.

Autoimmune enteropathy is a rare autoimmune disorder characterized by weight loss from malabsorption, severe and protracted diarrhea, and autoimmune damage to the intestinal mucosa. Autoimmune enteropathy typically occurs in infants and younger children however, adult cases have been reported in literature. Autoimmune enteropathy was first described by Walker-Smith et al. in 1982.

LRBA deficiency is a rare genetic disorder of the immune system. This disorder is caused by a mutation in the gene LRBA. LRBA stands for “lipopolysaccharide (LPS)-responsive and beige-like anchor protein”. This condition is characterized by autoimmunity, lymphoproliferation, and immune deficiency. It was first described by Gabriela Lopez-Herrera from University College London in 2012. Investigators in the laboratory of Dr. Michael Lenardo at National Institute of Allergy and Infectious Diseases, the National Institutes of Health and Dr. Michael Jordan at Cincinnati Children’s Hospital Medical Center later described this condition and therapy in 2015.

The intestinal mucosal barrier, also referred to as intestinal barrier, refers to the property of the intestinal mucosa that ensures adequate containment of undesirable luminal contents within the intestine while preserving the ability to absorb nutrients. The separation it provides between the body and the gut prevents the uncontrolled translocation of luminal contents into the body proper. Its role in protecting the mucosal tissues and circulatory system from exposure to pro-inflammatory molecules, such as microorganisms, toxins, and antigens is vital for the maintenance of health and well-being. Intestinal mucosal barrier dysfunction has been implicated in numerous health conditions such as: food allergies, microbial infections, irritable bowel syndrome, inflammatory bowel disease, celiac disease, metabolic syndrome, non-alcoholic fatty liver disease, diabetes, and septic shock.
References
- ↑ Wildin RS, Smyk-Pearson S, Filipovich AH (August 2002). "Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome". J Med Genet. 39 (8): 537–45. doi:10.1136/jmg.39.8.537. PMID 12161590.
- 1 2 Bacchetta, Rosa; Barzaghi, Federica; Roncarolo, Maria-Grazia (2018). "From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation". Annals of the New York Academy of Sciences. 1417 (1): 5–22. Bibcode:2018NYASA1417....5B. doi: 10.1111/nyas.13011 . ISSN 1749-6632. PMID 26918796.
- ↑ Bennett, Craig L.; Christie, Jacinda; Ramsdell, Fred; Brunkow, Mary E.; Ferguson, Polly J.; Whitesell, Luke; Kelly, Thaddeus E.; Saulsbury, Frank T.; Chance, Phillip F.; Ochs, Hans D. (January 2001). "The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3". Nature Genetics. 27 (1): 20–21. doi:10.1038/83713. ISSN 1546-1718. PMID 11137993. S2CID 205097191.
- 1 2 McGinness, Jamie L.; Bivens, Mary-Margaret C.; Greer, Kenneth E.; Patterson, James W.; Saulsbury, Frank T. (July 2006). "Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) associated with pemphigoid nodularis: A case report and review of the literature". Journal of the American Academy of Dermatology. 55 (1): 143–148. doi:10.1016/j.jaad.2005.08.047. ISSN 0190-9622. PMID 16781310.
- ↑ Villaseñor, Jennifer; Benoist, Christophe; Mathis, Diane (2005). "AIRE and APECED: molecular insights into an autoimmune disease". Immunological Reviews. 204 (1): 156–164. doi:10.1111/j.0105-2896.2005.00246.x. ISSN 1600-065X. PMID 15790357. S2CID 31003833.
- 1 2 3 Liston, Adrian; Enders, Anselm; Siggs, Owen M. (July 2008). "Unravelling the association of partial T-cell immunodeficiency and immune dysregulation". Nature Reviews Immunology. 8 (7): 545–558. doi: 10.1038/nri2336 . ISSN 1474-1741. PMID 18551129. S2CID 22248040.
- ↑ Schubert, Desirée; Bode, Claudia; Kenefeck, Rupert; Hou, Tie Zheng; Wing, James B.; Kennedy, Alan; Bulashevska, Alla; Petersen, Britt-Sabina; Schäffer, Alejandro A.; Grüning, Björn A.; Unger, Susanne (December 2014). "Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations". Nature Medicine. 20 (12): 1410–1416. doi:10.1038/nm.3746. ISSN 1546-170X. PMC 4668597 . PMID 25329329.
- 1 2 Fagundes, Christopher P.; Glaser, Ronald; Kiecolt-Glaser, Janice K. (2013-01-01). "Stressful early life experiences and immune dysregulation across the lifespan". Brain, Behavior, and Immunity. 27 (1): 8–12. doi:10.1016/j.bbi.2012.06.014. ISSN 0889-1591. PMC 3518756 . PMID 22771426.
- ↑ Godbout, Jonathan P.; Glaser, Ronald (2006-12-01). "Stress-Induced Immune Dysregulation: Implications for Wound Healing, Infectious Disease and Cancer". Journal of Neuroimmune Pharmacology. 1 (4): 421–427. doi:10.1007/s11481-006-9036-0. ISSN 1557-1904. PMID 18040814. S2CID 22738931.
- ↑ Kronfol, Ziad (December 2002). "Immune dysregulation in major depression: a critical review of existing evidence". The International Journal of Neuropsychopharmacology. 5 (4): 333–343. doi: 10.1017/s1461145702003024 . ISSN 1461-1457. PMID 12466033.
- 1 2 3 4 Ventura, Maria Teresa; Casciaro, Marco; Gangemi, Sebastiano; Buquicchio, Rosalba (December 2017). "Immunosenescence in aging: between immune cells depletion and cytokines up-regulation". Clinical and Molecular Allergy. 15 (1): 21. doi: 10.1186/s12948-017-0077-0 . ISSN 1476-7961. PMC 5731094 . PMID 29259496.
- 1 2 3 Fulop, Tamas; Witkowski, Jacek M.; Olivieri, Fabiola; Larbi, Anis (December 2018). "The integration of inflammaging in age-related diseases". Seminars in Immunology. 40: 17–35. doi:10.1016/j.smim.2018.09.003. ISSN 1044-5323. PMID 30287177. S2CID 52920736.
- 1 2 3 Bonafè, Massimiliano; Prattichizzo, Francesco; Giuliani, Angelica; Storci, Gianluca; Sabbatinelli, Jacopo; Olivieri, Fabiola (June 2020). "Inflamm-aging: Why older men are the most susceptible to SARS-CoV-2 complicated outcomes". Cytokine & Growth Factor Reviews. 53: 33–37. doi:10.1016/j.cytogfr.2020.04.005. ISSN 1359-6101. PMC 7252014 . PMID 32389499.
- 1 2 3 Gouin, Jean-Philippe; Hantsoo, Liisa; Kiecolt-Glaser, Janice K. (2008). "Immune Dysregulation and Chronic Stress among Older Adults: A Review". Neuroimmunomodulation. 15 (4–6): 251–259. doi:10.1159/000156468. ISSN 1423-0216. PMC 2676338 . PMID 19047802.
- 1 2 Mokarizadeh, Aram; Faryabi, Mohammad Reza; Rezvanfar, Mohammad Amin; Abdollahi, Mohammad (2015-05-04). "A comprehensive review of pesticides and the immune dysregulation: mechanisms, evidence and consequences". Toxicology Mechanisms and Methods. 25 (4): 258–278. doi:10.3109/15376516.2015.1020182. ISSN 1537-6516. PMID 25757504. S2CID 662060.
- 1 2 3 Palm, Noah W.; Rosenstein, Rachel K.; Medzhitov, Ruslan (April 2012). "Allergic host defences". Nature. 484 (7395): 465–472. Bibcode:2012Natur.484..465P. doi:10.1038/nature11047. ISSN 0028-0836. PMC 3596087 . PMID 22538607.
- 1 2 Yazdanbakhsh, M. (2002-04-19). "Allergy, Parasites, and the Hygiene Hypothesis". Science. 296 (5567): 490–494. Bibcode:2002Sci...296..490Y. doi:10.1126/science.296.5567.490. ISSN 0036-8075. PMID 11964470.
- ↑ Lambrecht, Bart N; Hammad, Hamida (October 2017). "The immunology of the allergy epidemic and the hygiene hypothesis". Nature Immunology. 18 (10): 1076–1083. doi:10.1038/ni.3829. ISSN 1529-2908. PMID 28926539. S2CID 6239349.