
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin.
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins.
Trypsinogen is the precursor form of trypsin, a digestive enzyme. It is produced by the pancreas and found in pancreatic juice, along with amylase, lipase, and chymotrypsinogen. It is cleaved to its active form, trypsin, by enteropeptidase, which is found in the intestinal mucosa. Once activated, the trypsin can cleave more trypsinogen into trypsin, a process called autoactivation. Trypsin cleaves the peptide bond on the carboxyl side of basic amino acids such as arginine and lysine.

Beta-secretase 2 is an enzyme that cleaves Glu-Val-Asn-Leu!Asp-Ala-Glu-Phe in the Swedish variant of Alzheimer's amyloid precursor protein. BACE2 is a close homolog of BACE1.
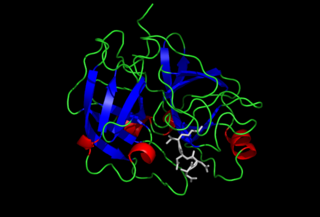
Enteropeptidase is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen into its active form trypsin, resulting in the subsequent activation of pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment.
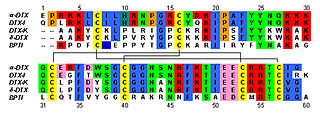
Dendrotoxins are a class of presynaptic neurotoxins produced by mamba snakes (Dendroaspis) that block particular subtypes of voltage-gated potassium channels in neurons, thereby enhancing the release of acetylcholine at neuromuscular junctions. Because of their high potency and selectivity for potassium channels, dendrotoxins have proven to be extremely useful as pharmacological tools for studying the structure and function of these ion channel proteins.
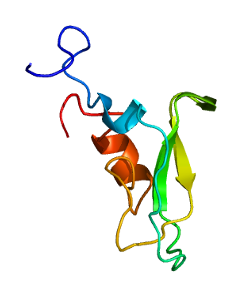
Tissue factor pathway inhibitor is a single-chain polypeptide which can reversibly inhibit factor Xa (Xa). While Xa is inhibited, the Xa-TFPI complex can subsequently also inhibit the FVIIa-tissue factor complex. TFPI contributes significantly to the inhibition of Xa in vivo, despite being present at concentrations of only 2.5 nM.
A trypsin inhibitor (TI) is a protein and a type of serine protease inhibitor (serpin) that reduces the biological activity of trypsin by controlling the activation and catalytic reactions of proteins. Trypsin is an enzyme involved in the breakdown of many different proteins, primarily as part of digestion in humans and other animals such as monogastrics and young ruminants. Serpins – including trypsin inhibitors – are irreversible and suicide substrate-like inhibitors.
The drug aprotinin, is a small protein bovine pancreatic trypsin inhibitor (BPTI), or basic trypsin inhibitor of bovine pancreas, which is an antifibrinolytic molecule that inhibits trypsin and related proteolytic enzymes. Under the trade name Trasylol, aprotinin was used as a medication administered by injection to reduce bleeding during complex surgery, such as heart and liver surgery. Its main effect is the slowing down of fibrinolysis, the process that leads to the breakdown of blood clots. The aim in its use was to decrease the need for blood transfusions during surgery, as well as end-organ damage due to hypotension as a result of marked blood loss. The drug was temporarily withdrawn worldwide in 2007 after studies suggested that its use increased the risk of complications or death; this was confirmed by follow-up studies. Trasylol sales were suspended in May 2008, except for very restricted research use. In February 2012 the European Medicines Agency (EMA) scientific committee reverted its previous standpoint regarding aprotinin, and has recommended that the suspension be lifted. Nordic became distributor of aprotinin in 2012.

Amyloid-like protein 2, also known as APLP2, is a protein that in humans is encoded by the APLP2 gene. APLP2 along with APLP1 are important modulators of glucose and insulin homeostasis.

Pancreatic secretory trypsin inhibitor (PSTI) also known as serine protease inhibitor Kazal-type 1 (SPINK1) or tumor-associated trypsin inhibitor (TATI) is a protein that in humans is encoded by the SPINK1 gene.

Kunitz-type protease inhibitor 2 is an enzyme inhibitor that in humans is encoded by the SPINT2 gene. SPINT2 is a transmembrane protein with two extracellular Kunitz domains to inhibit serine proteases. This gene is a presumed tumor suppressor by inhibiting HGF activator which prevents the formation of active hepatocyte growth factor. Mutations in SPINT2 could result in congenital sodium diarrhea (CSD).
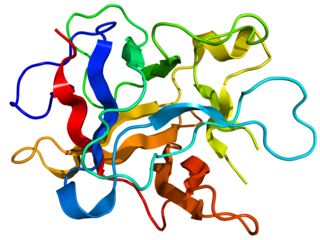
Kunitz soybean trypsin inhibitor is a type of protein contained in legume seeds which functions as a protease inhibitor. Kunitz-type Soybean Trypsin Inhibitors are usually specific for either trypsin or chymotrypsin. They are thought to protect seeds against consumption by animal predators.
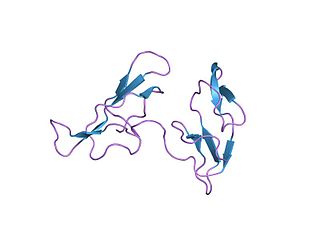
In molecular biology, the Bowman–Birk protease inhibitor family of proteins consists of eukaryotic proteinase inhibitors, belonging to MEROPS inhibitor family I12, clan IF. They mainly inhibit serine peptidases of the S1 family, but also inhibit S3 peptidases.

The Kazal domain is an evolutionary conserved protein domain usually indicative of serine protease inhibitors. However, kazal-like domains are also seen in the extracellular part of agrins, which are not known to be protease inhibitors.

Protease, serine, 3 is a protein that in humans is encoded by the PRSS3 gene.

WAP, follistatin/kazal, immunoglobulin, kunitz and netrin domain containing 2 is a protein that in humans is encoded by the WFIKKN2 gene.
Kalicludine (AsKC) is a blocker of the voltage-dependent potassium channel Kv1.2 found in the snakeslocks anemone Anemonia viridis, which it uses to paralyse prey.

Cry6Aa is a toxic crystal protein generated by the bacterial family Bacillus thuringiensis during sporulation. This protein is a member of the alpha pore forming toxins family, which gives it insecticidal qualities advantageous in agricultural pest control. Each Cry protein has some level of target specificity; Cry6Aa has specific toxic action against coleopteran insects and nematodes. The corresponding B. thuringiensis gene, cry6aa, is located on bacterial plasmids. Along with several other Cry protein genes, cry6aa can be genetically recombined in Bt corn and Bt cotton so the plants produce specific toxins. Insects are developing resistance to the most commonly inserted proteins like Cry1Ac. Since Cry6Aa proteins function differently than other Cry proteins, they are combined with other proteins to decrease the development of pest resistance. Recent studies suggest this protein functions better in combination with other virulence factors such as other Cry proteins and metalloproteinases.>
















