An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen, via the Fab's variable region. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize its target directly. Depending on the antigen, the binding may impede the biological process causing the disease or may activate macrophages to destroy the foreign substance. The ability of an antibody to communicate with the other components of the immune system is mediated via its Fc region, which contains a conserved glycosylation site involved in these interactions. The production of antibodies is the main function of the humoral immune system.
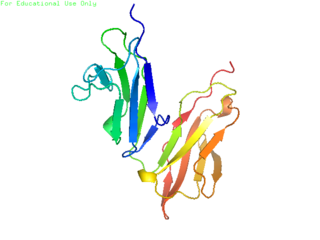
CD32 is a surface receptor protein and part of a large population of B cell co-receptors, which act to modulate signaling.
Humanized antibodies are antibodies from non-human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans. The process of "humanization" is usually applied to monoclonal antibodies developed for administration to humans. Humanization can be necessary when the process of developing a specific antibody involves generation in a non-human immune system. The protein sequences of antibodies produced in this way are partially distinct from homologous antibodies occurring naturally in humans, and are therefore potentially immunogenic when administered to human patients. There are other types of antibodies developed. The International Nonproprietary Names of humanized antibodies end in -zumab, as in omalizumab.

Protein A is a 42 kDa surface protein originally found in the cell wall of the bacteria Staphylococcus aureus. It is encoded by the spa gene and its regulation is controlled by DNA topology, cellular osmolarity, and a two-component system called ArlS-ArlR. It has found use in biochemical research because of its ability to bind immunoglobulins. It is composed of five homologous Ig-binding domains that fold into a three-helix bundle. Each domain is able to bind proteins from many mammalian species, most notably IgGs. It binds the heavy chain within the Fc region of most immunoglobulins and also within the Fab region in the case of the human VH3 family. Through these interactions in serum, where IgG molecules are bound in the wrong orientation, the bacteria disrupts opsonization and phagocytosis.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAb) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. More recently antibodies have been used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
Biological response modifiers (BRMs) are substances that modify immune responses. They can be both endogenous and exogenous, and they can either enhance an immune response or suppress it. Some of these substances arouse the body's response to an infection, and others can keep the response from becoming excessive. Thus they serve as immunomodulators in immunotherapy, which can be helpful in treating cancer and in treating autoimmune diseases, such as some kinds of arthritis and dermatitis. Most BRMs are biopharmaceuticals (biologics), including monoclonal antibodies, interleukin 2, interferons, and various types of colony-stimulating factors. "Immunotherapy makes use of BRMs to enhance the activity of the immune system to increase the body's natural defense mechanisms against cancer", whereas BRMs for rheumatoid arthritis aim to reduce inflammation.
Abatacept is a drug used to treat autoimmune diseases like rheumatoid arthritis, by interfering with the immune activity of T cells. It is a modified antibody.
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophil polymorphonuclear leukocytes, monocytes and macrophages. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.

In immunology, the immunoglobulin (Ig) isotype (class) is encoded by the constant region segments of the immunoglobulin gene which form the Fc portion of an antibody. The expression of a specific isotype determines the function of an antibody via the specific binding to Fc receptor molecules on different immune effector cells. Isotype expression reflects the maturation stage of a B cell. Naive B cells express IgM and IgD isotypes with unmutated variable genes, which are produced from the same initial transcript following alternative splicing. Expression of other antibody isotypes occurs via a process of class-switch recombination (CSR) after antigen exposure. Class-switching is mediated by the AID enzyme and only occurs after the B cell binds an antigen through its B cell receptor, and is further activated through interaction with a T helper cell.
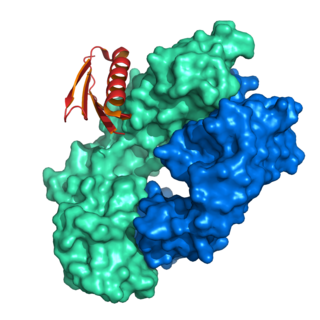
Protein L was first isolated from the surface of bacterial species Peptostreptococcus magnus and was found to bind immunoglobulins through L chain interaction, from which the name was suggested. It consists of 719 amino acid residues. The molecular weight of Protein L purified from the cell walls of Peptostreptoccus magnus was first estimated as 95kD by SDS-PAGE in the presence of reducing agent 2-mercaptoethanol, while the molecular weight was determined to 76kD by gel chromotography in the presence of 6 M guanidine HCl. Protein L does not contain any interchain disulfide loops, nor does it consist of disulfide-linked subunits. It is an acidic molecule with a pI of 4.0. Unlike Protein A and Protein G, which bind to the Fc region of immunoglobulins (antibodies), Protein L binds antibodies through light chain interactions. Since no part of the heavy chain is involved in the binding interaction, Protein L binds a wider range of antibody classes than Protein A or G. Protein L binds to representatives of all antibody classes, including IgG, IgM, IgA, IgE and IgD. Single chain variable fragments (scFv) and Fab fragments also bind to Protein L.
Trubion was a publicly held biopharmaceutical company that was focused on creating a pipeline of protein-based therapeutic product candidates to treat autoimmune and inflammatory diseases and cancer. Trubion was acquired by Emergent BioSolutions on October 28, 2010. Trubion was established in 1999 in the State of Washington as an early stage development company, and was later reincorporated in October 2002 in the State of Delaware.
Atacicept is a recombinant fusion protein designed to inhibit B cells, thereby suppressing autoimmune disease. The designer protein combines the binding site for two cytokines that regulate maturation, function, and survival of B cells - B-lymphocyte stimulator (BLyS) and A proliferation-inducing ligand (APRIL), with the constant region of immunoglobin. Atacicept blocks activation of B cells by the tumor necrosis factor receptor superfamily member 13B, a transmembrane receptor protein found predominantly on the surface of B cells. Like the monoclonal antibody belimumab, atacicept blocks the binding of BLyS, but it also blocks APRIL. Binding of these TACI ligands induces proliferation, activation, and longevity of B cells and thus their production of autoantibodies. Atacicept is thought to selectively impair mature B cells and plasma cells with less impact on progenitor cells and memory B cells.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.
Ocaratuzumab is a humanized monoclonal antibody designed for the treatment of cancer and autoimmune disorders. The antibody is engineered for enhanced affinity to the CD20 antigen on B-lymphocytes, increased antibody-dependent cell-mediated cytotoxicity (ADCC), and for improved treatment of low-affinity FcγRIIIa allotypes.

ImmTACs are a class of bispecific biological drug being investigated for the treatment of cancer and viral infections which combines engineered cancer-recognizing TCRs with immune activating complexes. ImmTACs target cancerous or virally infected cells through binding human leukocyte antigen (HLA) presented peptide antigens and redirect the host's cytotoxic T cells to recognise and kill them.
Recombinant antibodies are antibody fragments produced by using recombinant antibody coding genes. They mostly consist of a heavy and light chain of the variable region of immunoglobulin. Recombinant antibodies have many advantages in both medical and research applications, which make them a popular subject of exploration and new production against specific targets. The most commonly used form is the single chain variable fragment (scFv), which has shown the most promising traits exploitable in human medicine and research. In contrast to monoclonal antibodies produced by hybridoma technology, which may lose the capacity to produce the desired antibody over time or the antibody may undergo unwanted changes, which affect its functionality, recombinant antibodies produced in phage display maintain high standard of specificity and low immunogenicity.

MOR103 is a so-called fully human antibody which has been developed by the biotechnology company MorphoSys. It can also be referred to as HuCAL antibody, HuCAL standing for Human Combinatorial Antibody Library and being a technology used to generate monoclonal antibodies. MOR103 is directed against the granulocyte-macrophage colony stimulating factor (GM-CSF), a monomeric glycoprotein functioning as a cytokine promoting both proliferation and activation of macrophages and neutrophils.