Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.

Cluster of differentiation 40, CD40 is a type I transmembrane protein found on antigen-presenting cells and is required for their activation. The binding of CD154 (CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of downstream effects.
Bapineuzumab is a humanized monoclonal antibody that acts on the nervous system and may have potential therapeutic value for the treatment of Alzheimer's disease and possibly glaucoma. However, in 2012 it failed to produce significant cognitive improvements in patients in two major trials, despite lowering key biomarkers of AD, amyloid brain plaque and hyperphosphorylated tau protein in CSF.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.

CD137, a member of the tumor necrosis factor (TNF) receptor family, is a type 1 transmembrane protein, expressed on surfaces of leukocytes and non-immune cells. Its alternative names are tumor necrosis factor receptor superfamily member 9 (TNFRSF9), 4-1BB, and induced by lymphocyte activation (ILA). It is of interest to immunologists as a co-stimulatory immune checkpoint molecule, and as a potential target in cancer immunotherapy.
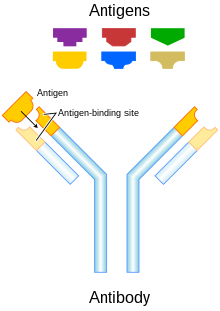
A bispecific monoclonal antibody is an artificial protein that can simultaneously bind to two different types of antigen or two different epitopes on the same antigen. Naturally occurring antibodies typically only target one antigen. BsAbs can be manufactured in several structural formats. BsAbs can be designed to recruit and activate immune cells, to interfere with receptor signaling and inactivate signaling ligands, and to force association of protein complexes. BsAbs have been explored for cancer immunotherapy, drug delivery, and Alzheimer's disease.
Solanezumab is a monoclonal antibody being investigated by Eli Lilly as a neuroprotector for patients with Alzheimer's disease. The drug originally attracted extensive media coverage proclaiming it a breakthrough, but it has failed to show promise in Phase III trials.

A trifunctional antibody is a monoclonal antibody with binding sites for two different antigens, typically CD3 and a tumor antigen, making it a type of bispecific monoclonal antibody. In addition, its intact Fc-part can bind to an Fc receptor on accessory cells like conventional monospecific antibodies. The net effect is that this type of drug links T cells and monocytes/macrophages, natural killer cells, dendritic cells or other Fc receptor expressing cells to the tumor cells, leading to their destruction.
Active immunotherapy is a type of immunotherapy that aims to stimulate the host's immune system or a specific immune response to a disease or pathogen and is most commonly used in cancer treatments. Active immunotherapy is also used for treatment of neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, prion disease, and multiple sclerosis. Active immunotherapies induce an immune response through direct immune system stimulation, while immunotherapies that administer antibodies directly to the system are classified as passive immunotherapies. Active immunotherapies can elicit generic and specific immune responses depending on the goal of the treatment. The categories of active immunotherapy divide into:
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.
Racotumomab is a therapeutic cancer vaccine for the treatment of solid tumors that is currently under clinical development by ReComBio, an international public-private consortium with the participation of the Center of Molecular Immunology at Havana, Cuba (CIM) and researchers from Buenos Aires University and National University of Quilmes in Argentina. It induces the patient's immune system to generate a response against a cancer-specific molecular target with the purpose of blocking tumor growth, slowing disease progression and ultimately increasing patient survival.

Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.
MDX-1097 is a monoclonal antibody therapy that in 2023 has been assessed in a Phase IIb clinical trial in conjunction with lenalidomide and dexamethasone as a treatment for multiple myeloma, a type of white blood cell cancer. MDX-1097 was originally developed by scientists at Immune System Therapeutics Ltd. In 2015, Haemalogix Ltd acquired the rights to MDX-1097 and are taking it through clinical testing.

Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately. However, some cancers can protect themselves from attack by stimulating immune checkpoint targets.
Lecanemab, sold under the brand name Leqembi, is a monoclonal antibody medication used for the treatment of Alzheimer's disease. Lecanemab is an amyloid beta-directed antibody. It is given via intravenous infusion. The most common side effects of lecanemab include headache, infusion-related reactions, and amyloid-related imaging abnormalities, a side effect known to occur with the class of antibodies targeting amyloid.
Dostarlimab, sold under the brand name Jemperli, is a monoclonal antibody used as an anti-cancer medication for the treatment of endometrial cancer. Dostarlimab is a programmed death receptor-1 (PD-1)–blocking monoclonal antibody.
Donanemab is a biological drug in Phase III clinical trials to determine whether it slows the progression of early Alzheimer's disease. Donanemab has shown positive results in its first trials. Donanemab was developed by the Eli Lilly and Co. and is under clinical development as a possible treatment for Alzheimer's disease. There is currently no approved cure or disease-modifying treatment for Alzheimer's disease except for lecanemab.
Passive antibody therapy, also called serum therapy, is a subtype of passive immunotherapy that administers antibodies to target and kill pathogens or cancer cells. It is designed to draw support from foreign antibodies that are donated from a person, extracted from animals, or made in the laboratory to elicit an immune response instead of relying on the innate immune system to fight disease. It has a long history from the 18th century for treating infectious diseases and is now a common cancer treatment. The mechanism of actions include: antagonistic and agonistic reaction, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC).
Oligoclonal antibodies are an emerging immunological treatment relying on the combinatory use of several monoclonal antibodies (mAb) in one single drug. The composition can be made of mAb targeting different epitopes of a same protein (homo-combination) or mAb targeting different proteins (hetero-combination). It mimicks the natural polyclonal humoral immunological response to get better efficiency of the treatment. This strategy is most efficient in infections and in cancer treatment as it allow to overcome acquired resistance by pathogens and the plasticity of cancers.
Anti-amyloid drugs, also known as anti-amyloid antibodies (AAA), are a class of monoclonal antibodies developed to treat Alzheimer's disease. The first drug in the class to be developed, in the early 2000s, was bapineuzumab, but it did not show effectiveness in later-stage trials. The first drug to be FDA approved was aducanumab in 2021.