
Cholangiocarcinoma, also known as bile duct cancer, is a type of cancer that forms in the bile ducts. Symptoms of cholangiocarcinoma may include abdominal pain, yellowish skin, weight loss, generalized itching, and fever. Light colored stool or dark urine may also occur. Other biliary tract cancers include gallbladder cancer and cancer of the ampulla of Vater.

Lenvatinib, sold under the brand name Lenvima among others, is an anti-cancer medication for the treatment of certain kinds of thyroid cancer and for other cancers as well. It was developed by Eisai Co. and acts as a multiple kinase inhibitor against the VEGFR1, VEGFR2 and VEGFR3 kinases.

Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.
Margetuximab, sold under the brand name Margenza, is a chimeric IgG monoclonal antibody medication against HER2 used for the treatment of cancer.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is given by slow injection into a vein.
Enfortumab vedotin, sold under the brand name Padcev, is an antibody-drug conjugate used for the treatment of urothelial cancer. It is a nectin-4-directed antibody and microtubule inhibitor conjugate. Enfortumab refers to the monoclonal antibody part, and vedotin refers to the payload drug (MMAE) and the linker.

Atezolizumab, sold under the brand name Tecentriq, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma and alveolar soft part sarcoma, but discontinued for use in triple-negative breast cancer (TNBC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).

Sacituzumab govitecan, sold under the brand name Trodelvy, is a Trop-2-directed antibody and topoisomerase inhibitor drug conjugate used for the treatment of metastatic triple-negative breast cancer and metastatic urothelial cancer.
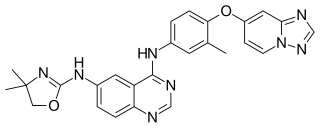
Tucatinib, sold under the brand name Tukysa, is an anticancer medication used for the treatment of HER2-positive breast cancer. It is a small molecule inhibitor of HER2. It was developed by Array BioPharma and licensed to Cascadian Therapeutics.

Erdafitinib, sold under the brand name Balversa, is an anti-cancer medication. It is a small molecule inhibitor of fibroblast growth factor receptor (FGFR) used for the treatment of cancer. FGFRs are a subset of tyrosine kinases which are unregulated in some tumors and influence tumor cell differentiation, proliferation, angiogenesis, and cell survival. Astex Pharmaceuticals discovered the drug and licensed it to Janssen Pharmaceuticals for further development.

Ivosidenib, sold under the brand name Tibsovo, is an anti-cancer medication for the treatment of acute myeloid leukemia (AML) and cholangiocarcinoma. It is a small molecule inhibitor of isocitrate dehydrogenase-1 (IDH1), which is mutated in several forms of cancer. Ivosidenib is an isocitrate dehydrogenase-1 inhibitor that works by decreasing abnormal production of the oncometabolite 2-hydroxyglutarate (2-HG), leading to differentiation of malignant cells.

Tepotinib, sold under the brand name Tepmetko, is an anti-cancer medication used for the treatment of adults with non-small cell lung cancer (NSCLC).
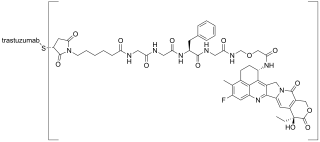
Trastuzumab deruxtecan, sold under the brand name Enhertu, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the topoisomerase I inhibitor deruxtecan. It is licensed for the treatment of breast cancer or gastric or gastroesophageal adenocarcinoma. Trastuzumab binds to and blocks signaling through epidermal growth factor receptor 2 (HER2/neu) on cancers that rely on it for growth. Additionally, once bound to HER2 receptors, the antibody is internalized by the cell, carrying the bound deruxtecan along with it, where it interferes with the cell's ability to make DNA structural changes and replicate its DNA during cell division, leading to DNA damage when the cell attempts to replicate itself, destroying the cell.

Pemigatinib, sold under the brand name Pemazyre, is an anti-cancer medication used for the treatment of bile duct cancer (cholangiocarcinoma). Pemigatinib works by blocking FGFR2 in tumor cells to prevent them from growing and spreading.

Selpercatinib, sold under the brand name Retevmo among others, is a medication for the treatment of cancers in people whose tumors have an alteration in a specific gene. It is taken by mouth.

Ripretinib, sold under the brand name Qinlock, is a medication for the treatment of adults with advanced gastrointestinal stromal tumor (GIST), a type of tumor that originates in the gastrointestinal tract. It is taken by mouth. Ripretinib inhibits the activity of the kinases KIT and PDGFRA, which helps keep cancer cells from growing.
Pralsetinib, sold under the brand name Gavreto, is a medication approved for RET mutation-positive medullary thyroid cancer (MTC) and RET fusion-positive differentiated thyroid cancer (DTC) refractory to radioactive iodine (RAI) therapy. Pralsetinib is a tyrosine kinase inhibitor. It is taken by mouth.
Tebentafusp, sold under the brand name Kimmtrak, is an anti-cancer medication used to treat uveal melanoma. Tebentafusp is a bispecific gp100 peptide-HLA-directed CD3 T cell engager. Tebentafusp is given by intravenous infusion.

Futibatinib, sold under the brand name Lytgobi, is an anti-cancer medication used for the treatment of cholangiocarcinoma. It is a kinase inhibitor. It is taken by mouth.

Fruquintinib, sold under the brand name Fruzaqla, is an anti-cancer medication used for the treatment of colorectal cancer. Fruquintinib is a kinase inhibitor.
















