Epiregulin (EPR) is a protein that in humans is encoded by the EREG gene. [5] [6]
Epiregulin (EPR) is a protein that in humans is encoded by the EREG gene. [5] [6]
Epiregulin consists of 46 amino acid residues. Its secondary structure contains approximately 30 percent of β-sheet in the strand. [7] Some of the residues form loops and turns due to the hydrogen bonding. [7] The percentage of β-sheet in epiregulin depends on the domain and the secondary structures that they occupy. The polymeric molecules of epiregulin has the formula weight of 5280.1 g/mol with a polypeptide(L), a polymer type. [7]
Structural motifs in most proteins have typical connections in an all β motif. Meaning that the polypeptide chains do not make a crossover connection or in so far as this type of connection has not been observed. Epiregulin is one of the proteins that occupies a typical connection in all β motif. Furthermore, as the structure of epiregulin forms a chain in an all β motif, it also forms β hairpin structural motif. A β hairpin is when the two adjacent anti-parallel β strands connected by a β-turn.
Epiregulin is a member of the epidermal growth factor family. Epiregulin can function as a ligand of epidermal growth factor receptor (EGFR), as well as a ligand of most members of the ERBB (v-erb-b2 oncogene homolog) family of tyrosine-kinase receptors. [6] The secondary structure at the C-terminus epiregulin is different from other epidermal growth factor family ligands because of the lack of hydrogen bonds. The structural difference at the C-terminus may provide an explanation for the reduced binding affinity of epiregulin to the ERBB receptors. [7]
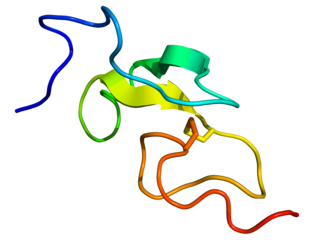
Epidermal growth factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is 6-kDa and has 53 amino acid residues and three intramolecular disulfide bonds.

Amphiregulin, also known as AREG, is a protein synthesized as a transmembrane glycoprotein with 252 aminoacids and it is encoded by the AREG gene. in humans.
The MAPK/ERK pathway is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell.

Mothers against decapentaplegic homolog 7 or SMAD7 is a protein that in humans is encoded by the SMAD7 gene.

Sorting nexin-1 is a protein that in humans is encoded by the SNX1 gene. The protein encoded by this gene is a sorting nexin. SNX1 is a component of the retromer complex.

Transforming growth factor alpha (TGF-α) is a protein that in humans is encoded by the TGFA gene. As a member of the epidermal growth factor (EGF) family, TGF-α is a mitogenic polypeptide. The protein becomes activated when binding to receptors capable of protein kinase activity for cellular signaling.
Cripto is an EGF-CFC or epidermal growth factor-CFC, which is encoded by the Cryptic family 1 gene. Cryptic family protein 1B is a protein that in humans is encoded by the CFC1B gene. Cryptic family protein 1B acts as a receptor for the TGF beta signaling pathway. It has been associated with the translation of an extracellular protein for this pathway. The extracellular protein which Cripto encodes plays a crucial role in the development of left and right division of symmetry.

Betaglycan also known as Transforming growth factor beta receptor III (TGFBR3), is a cell-surface chondroitin sulfate / heparan sulfate proteoglycan >300 kDa in molecular weight. Betaglycan binds to various members of the TGF-beta superfamily of ligands via its core protein, and bFGF via its heparan sulfate chains. It is not involved directly in TGF-beta signal transduction but by binding to various member of the TGF-beta superfamily at the cell surface it acts as a reservoir of ligand for TGF-beta receptors.
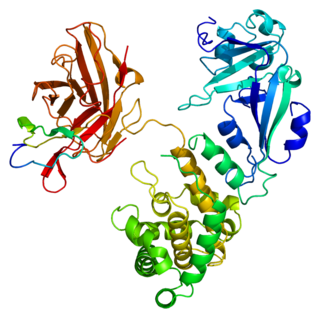
Heparin-binding EGF-like growth factor (HB-EGF) is a member of the EGF family of proteins that in humans is encoded by the HBEGF gene.

Betacellulin is a protein that in humans is encoded by the BTC gene located on chromosome 4 at locus 4q13-q21. Betacellulin was initially identified as a mitogen. Betacellulin, is a part of an Epidermal Growth Factor (EGF) family and functions as a ligand for the epidermal growth factor receptor (EGFR). As the role a EGFR, betacellulin is manifested by different form of muscles and tissues, it also has a great effect of nitrogen that is used for retinal pigment epithelial cells and vascular smooth muscle cells. While many studies attest a role for betacellulin in the differentiation of pancreatic β-cells, the last decade witnessed the association of betacellulin with many additional biological processes, ranging from reproduction to the control of neural stem cells. Betacellulin is a member of the EGF family of growth factors. It is synthesized primarily as a transmembrane precursor, which is then processed to mature molecule by proteolytic events.

Receptor tyrosine-protein kinase erbB-3, also known as HER3, is a membrane bound protein that in humans is encoded by the ERBB3 gene.

Receptor tyrosine-protein kinase erbB-4 is an enzyme that in humans is encoded by the ERBB4 gene. Alternatively spliced variants that encode different protein isoforms have been described; however, not all variants have been fully characterized.
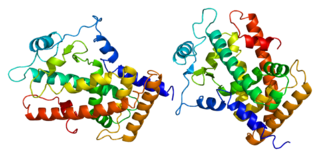
Peroxisome proliferator-activated receptor beta or delta, also known as NR1C2 is a nuclear receptor that in humans is encoded by the PPARD gene.

Cadherin EGF LAG seven-pass G-type receptor 3 is a protein that in humans is encoded by the CELSR3 gene.

Cadherin EGF LAG seven-pass G-type receptor 2 is a protein that in humans is encoded by the CELSR2 gene.

Low-density lipoprotein receptor-related protein 6 is a protein that in humans is encoded by the LRP6 gene. LRP6 is a key component of the LRP5/LRP6/Frizzled co-receptor group that is involved in canonical Wnt pathway.

Latent-transforming growth factor beta-binding protein 3 is a protein that in humans is encoded by the LTBP3 gene.

Fibroblast growth factor receptor substrate 3 is a protein that in humans is encoded by the FRS3 gene.

Stabilin-1 is a protein that in humans is encoded by the STAB1 gene.

Nardilysin is a protein that in humans is encoded by the NRD1 gene.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.