Platelet-derived growth factor (PDGF) is one among numerous growth factors that regulate cell growth and division. In particular, PDGF plays a significant role in blood vessel formation, the growth of blood vessels from already-existing blood vessel tissue, mitogenesis, i.e. proliferation, of mesenchymal cells such as fibroblasts, osteoblasts, tenocytes, vascular smooth muscle cells and mesenchymal stem cells as well as chemotaxis, the directed migration, of mesenchymal cells. Platelet-derived growth factor is a dimeric glycoprotein that can be composed of two A subunits (PDGF-AA), two B subunits (PDGF-BB), or one of each (PDGF-AB).

Platelet-derived growth factor receptors (PDGF-R) are cell surface tyrosine kinase receptors for members of the platelet-derived growth factor (PDGF) family. PDGF subunits -A and -B are important factors regulating cell proliferation, cellular differentiation, cell growth, development and many diseases including cancer. There are two forms of the PDGF-R, alpha and beta each encoded by a different gene. Depending on which growth factor is bound, PDGF-R homo- or heterodimerizes.
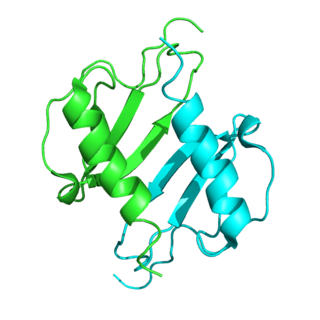
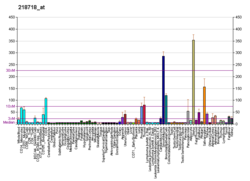
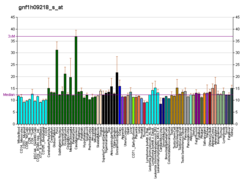
C-X-C motif chemokine 5 is a protein that in humans is encoded by the CXCL5 gene.

Chemokine ligand 7 (CXCL7) is a human gene.

The platelet-activating factor receptor(PAF-R) is a G-protein coupled receptor which binds platelet-activating factor. It is encoded in the human by the PTAFR gene.

Platelet-derived growth factor receptor beta is a protein that in humans is encoded by the PDGFRB gene. Mutations in PDGFRB are mainly associated with the clonal eosinophilia class of malignancies.

Platelet-derived growth factor subunit A is a protein that in humans is encoded by the PDGFA gene.

Protease activated receptor 3 (PAR-3) also known as coagulation factor II receptor-like 2 (F2RL2) and thrombin receptor-like 2, is a protein that in humans is encoded by the F2RL2 gene.

Platelet-derived growth factor subunit B is a protein that in humans is encoded by the PDGFB gene.

TYMP is a gene that encodes for the enzyme thymidine phosphorylase. The TYMP gene is also known as ECGF1 and MNGIE due to its role in MNGIE syndrome.

Protease-activated receptor 4 (PAR-4), also known as coagulation factor II (thrombin) receptor-like 3, is a protein that in humans is encoded by the F2RL3 gene.

Proto-oncogene tyrosine-protein kinase Yes is a non-receptor tyrosine kinase that in humans is encoded by the YES1 gene.

Interleukin 1 receptor, type I (IL1R1) also known as CD121a, is an interleukin receptor. IL1R1 also denotes its human gene.

Guanine nucleotide exchange factor VAV2 is a protein that in humans is encoded by the VAV2 gene.

Collagen alpha-1(XI) chain is a protein that in humans is encoded by the COL11A1 gene.

Platelet-derived growth factor D is a protein that in humans is encoded by the PDGFD gene.
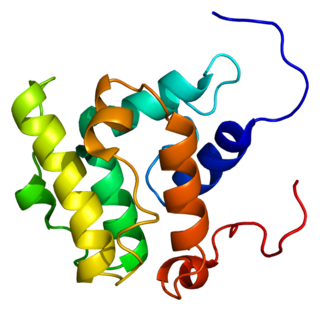
Guanine nucleotide exchange factor VAV3 is a protein that in humans is encoded by the VAV3 gene.

28 kDa heat- and acid-stable phosphoprotein is a protein that in humans is encoded by the PDAP1 gene.

Platelet-derived growth factor receptor A, also termed CD140a, is a receptor located on the surface of a wide range of cell types. The protein is encoded in the human by the PDGFRA gene. This receptor binds to certain isoforms of platelet-derived growth factors (PDGFs) and thereby becomes active in stimulating cell signaling pathways that elicit responses such as cellular growth and differentiation. The receptor is critical for the embryonic development of certain tissues and organs, and for their maintenance, particularly hematologic tissues, throughout life. Mutations in PDGFRA, are associated with an array of clinically significant neoplasms, notably ones of the clonal hypereosinophilia class of malignancies, as well as gastrointestinal stromal tumors (GISTs).
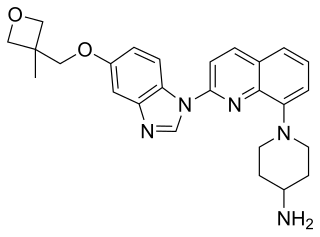
Crenolanib besylate is an investigational inhibitor being developed by AROG Pharmaceuticals, LLC. The compound is currently being evaluated for safety and efficacy in clinical trials for various types of cancer, including acute myeloid leukemia (AML), gastrointestinal stromal tumor (GIST), and glioma. Crenolanib is an orally bioavailable benzimidazole that selectively and potently inhibits signaling of wild-type and mutant isoforms of class III receptor tyrosine kinases (RTK) FLT3, PDGFR α, and PDGFR β. Unlike most RTK inhibitors, crenolanib is a type I mutant-specific inhibitor that preferentially binds to phosphorylated active kinases with the ‘DFG in’ conformation motif.