Ophthalmology is a surgical subspecialty within medicine that deals with the diagnosis and treatment of eye disorders. A former term is oculism.

Glaucoma is a group of eye diseases that lead to damage of the optic nerve, which transmits visual information from the eye to the brain. Glaucoma may cause vision loss if left untreated. It has been called the "silent thief of sight" because the loss of vision usually occurs slowly over a long period of time. A major risk factor for glaucoma is increased pressure within the eye, known as intraocular pressure (IOP). It is associated with old age, a family history of glaucoma, and certain medical conditions or medications. The word glaucoma comes from the Ancient Greek word γλαυκóς, meaning 'gleaming, blue-green, gray'.
The National Eye Institute (NEI) is part of the U.S. National Institutes of Health (NIH), an agency of the U.S. Department of Health and Human Services. The mission of NEI is "to eliminate vision loss and improve quality of life through vision research." NEI consists of two major branches for research: an extramural branch that funds studies outside NIH and an intramural branch that funds research on the NIH campus in Bethesda, Maryland. Most of the NEI budget funds extramural research.

Pilocarpine is a medication used to reduce pressure inside the eye and treat dry mouth. As an eye drop it is used to manage angle closure glaucoma until surgery can be performed, ocular hypertension, primary open angle glaucoma, and to constrict the pupil after dilation. However, due to its side effects it is no longer typically used for long-term management. Onset of effects with the drops is typically within an hour and lasts for up to a day. By mouth it is used for dry mouth as a result of Sjögren syndrome or radiation therapy.

Dry eye syndrome, also known as keratoconjunctivitis sicca, is the condition of having dry eyes. Symptoms include dryness in the eye, irritation, redness, discharge, blurred vision, and easily fatigued eyes. Symptoms range from mild and occasional to severe and continuous. Dry eye syndrome can lead to blurred vision, instability of the tear film, increased risk of damage to the ocular surface such as scarring of the cornea, and changes in the eye including the neurosensory system.

Timolol is a beta blocker medication used either by mouth or as eye drops. As eye drops it is used to treat increased pressure inside the eye such as in ocular hypertension and glaucoma. By mouth it is used for high blood pressure, chest pain due to insufficient blood flow to the heart, to prevent further complications after a heart attack, and to prevent migraines.
Ocular hypertension is the presence of elevated fluid pressure inside the eye, usually with no optic nerve damage or visual field loss.

Brimonidine is an α2 agonist medication used to treat open-angle glaucoma, ocular hypertension, and rosacea. In rosacea it improves the redness. It is used as eye drops or applied to the skin.

Tropomyosin receptor kinase A (TrkA), also known as high affinity nerve growth factor receptor, neurotrophic tyrosine kinase receptor type 1, or TRK1-transforming tyrosine kinase protein is a protein that in humans is encoded by the NTRK1 gene.
Tropomyosin receptor kinase C (TrkC), also known as NT-3 growth factor receptor, neurotrophic tyrosine kinase receptor type 3, or TrkC tyrosine kinase is a protein that in humans is encoded by the NTRK3 gene.
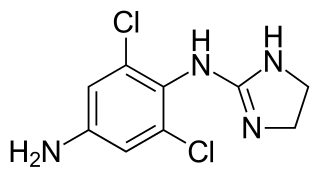
Apraclonidine (INN), also known under the brand name Iopidine, is a sympathomimetic used in glaucoma therapy. It is an α2 adrenergic receptor agonist and a weak α1 adrenergic receptor agonist.
Brimonidine/timolol, sold under the brand name Combigan among others, is a fixed-dose combination medication eye drop used for the treatment of glaucoma. It is a combination of brimonidine and timolol.

Lifitegrast, sold under the brand name Xiidra, is a medication for the treatment of signs and symptoms of dry eye, a syndrome called keratoconjunctivitis sicca. Lifitegrast reduces inflammation by inhibiting inflammatory cell binding. It is often used in conjunction with ciclosporin for dry eye treatment including meibomian gland dysfunction and inflammatory dry eye.

BNN-20, also known as 17β-spiro-(androst-5-en-17,2'-oxiran)-3β-ol, is a synthetic neurosteroid, "microneurotrophin", and analogue of the endogenous neurosteroid dehydroepiandrosterone (DHEA). It acts as a selective, high-affinity, centrally active agonist of the TrkA, TrkB, and p75NTR, receptors for the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), as well as for DHEA and DHEA sulfate (DHEA-S). The drug has been suggested as a potential novel treatment for Parkinson's disease and other conditions.

BNN-27, also known as 17α,20R-epoxypregn-5-ene-3β,21-diol, is a synthetic neurosteroid and "microneurotrophin" and analogue of the endogenous neurosteroid dehydroepiandrosterone (DHEA). It acts as a selective, high-affinity, centrally active agonist of the TrkA and p75NTR, receptors for nerve growth factor (NGF) and other neurotrophins, as well as for DHEA and DHEA sulfate (DHEA-S). BNN-27 has neuroprotective and neurogenic effects and has been suggested as a potential novel treatment for neurodegenerative diseases and brain trauma.
Cenegermin, sold under the brand name Oxervate, also known as recombinant human nerve growth factor (rhNGF), is a recombinant form of human nerve growth factor (NGF). In July 2017, it was approved in the European Union as an eye drop formulation for the treatment of moderate or severe neurotrophic keratitis in adults.
Michael Belkin is an Israeli academic and researcher working in ophthalmology, Professor Emeritus of Ophthalmology at Tel Aviv University. His research brought about advances in glaucoma treatment such as the ExPress glaucoma implant, the Ioptimate CO2 laser glaucoma surgery and a fast, non-contact glaucoma laser treatment.

Omidenepag, sold under the brand name Eybelis among others, is a medication used for the treatment of glaucoma and ocular hypertension.
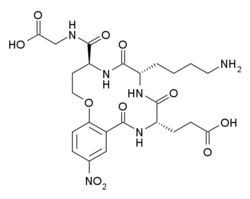
Neurotrophin mimetics are small molecules or peptide like molecules that can modulate the action of the neurotrophin receptor. One of the main causes of neurodegeneration involves changes in the expression of neurotrophins (NTs) and/or their receptors. Indeed, these imbalances or changes in their activity, lead to neuronal damage resulting in neurological and neurodegenerative conditions. The therapeutic properties of neurotrophins attracted the focus of many researchers during the years, but the poor pharmacokinetic properties, such as reduced bioavailability and low metabolic stability, the hyperalgesia, the inability to penetrate the blood–brain barrier and the short half-lives render the large neurotrophin proteins not suitable to be implemented as drugs.