
Insulin-like growth factor 1 (IGF-1), also called somatomedin C, is a hormone similar in molecular structure to insulin which plays an important role in childhood growth, and has anabolic effects in adults.

A fasciculation, or muscle twitch, is a spontaneous, involuntary muscle contraction and relaxation, involving fine muscle fibers. They are common, with as many as 70% of people experiencing them. They can be benign, or associated with more serious conditions. When no cause or pathology is identified, they are diagnosed as benign fasciculation syndrome.

Omigapil is a drug that was developed by Novartis and tested in clinical trials for its ability to help treat Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS). The development for PD and ALS have been terminated due to lack of benefit, but Santhera Pharmaceuticals bought the compound for development for the treatment of congenital muscular dystrophy (CMD).
Primary lateral sclerosis (PLS) is a very rare neuromuscular disease characterized by progressive muscle weakness in the voluntary muscles. PLS belongs to a group of disorders known as motor neuron diseases. Motor neuron diseases develop when the nerve cells that control voluntary muscle movement degenerate and die, causing weakness in the muscles they control.

Progressive muscular atrophy (PMA), also called Duchenne–Aran disease and Duchenne–Aran muscular atrophy, is a disorder characterised by the degeneration of lower motor neurons, resulting in generalised, progressive loss of muscle function.
Progressive bulbar palsy (PBP) is a medical condition. It belongs to a group of disorders known as motor neuron diseases. PBP is a disease that attacks the nerves supplying the bulbar muscles. These disorders are characterized by the degeneration of motor neurons in the cerebral cortex, spinal cord, brain stem, and pyramidal tracts. This specifically involves the glossopharyngeal nerve (IX), vagus nerve (X), and hypoglossal nerve (XII).
Gusperimus is an immunosuppressive drug. It is a derivative of the naturally occurring HSP70 inhibitor spergualin, and inhibits the interleukin-2-stimulated maturation of T cells to the S and G2/M phases and the polarization of the T cells into IFN-gamma-secreting Th1 effector T cells, resulting in the inhibition of growth of activated naive CD4 T cells.
Laron syndrome (LS), also known as growth hormone insensitivity or growth hormone receptor deficiency (GHRD), is an autosomal recessive disorder characterized by a lack of insulin-like growth factor 1 production in response to growth hormone. It is usually caused by inherited growth hormone receptor (GHR) mutations.

The ALS Association is an American nonprofit organization that funds global amyotrophic lateral sclerosis (ALS) research, provides care services and programs to people affected by ALS through its nationwide network of chapters, and works with ALS advocates around the country for state and federal policies that serve people living with amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease. The ALS Association is broken up into distinct chapters, each servicing a particular geographic area of the United States and all working under the umbrella of a national charter and administrator.
Mecasermin, sold under the brand name Increlex, also known as recombinant human insulin-like growth factor-1 (rhIGF-1), is a recombinant form of human insulin-like growth factor 1 (IGF-I) which is used in the long-term treatment of growth failure and short stature in children with severe primary IGF-I deficiency, for instance due to growth hormone deficiency or Laron syndrome.
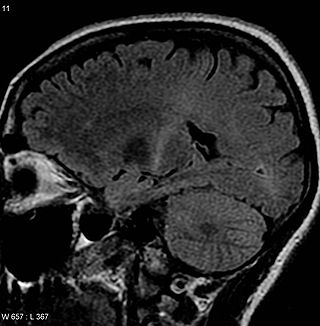
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND) or Lou Gehrig's disease, is a neurodegenerative disease that results in the progressive loss of motor neurons that control voluntary muscles. ALS is the most common form of the motor neuron diseases. Early symptoms of ALS include stiff muscles, muscle twitches, and gradual increasing weakness and muscle wasting. Limb-onset ALS begins with weakness in the arms or legs, while bulbar-onset ALS begins with difficulty speaking or swallowing. Around half of people with ALS develop at least mild difficulties with thinking and behavior, and about 15% develop frontotemporal dementia. Motor neuron loss continues until the ability to eat, speak, move, and finally the ability to breathe is lost with the cause of early death usually being respiratory failure.

Edaravone, sold under the brand name Radicava among others, is a medication used to treat stroke and amyotrophic lateral sclerosis (ALS). It is given by intravenous infusion and by mouth.
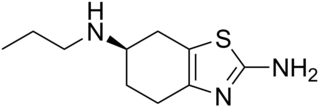
Dexpramipexole (KNS-760704) is an orally administered drug candidate shown to selectively and significantly lower eosinophil counts in human blood and tissue. The drug is currently in clinical development for eosinophil-associated diseases by Knopp Biosciences LLC.

Helixmith Co., Ltd. is a biotechnology company located in Seoul, Korea with US presence in San Diego. The company has an extensive gene therapy pipeline, including a non-viral plasmid DNA program for neuromuscular and ischemic disease, a CAR-T program targeting several different types of solid tumors, and an AAV vector program targeting neuromuscular diseases. Helixmith’s lead gene is Engensis (VM202), currently in phase III diabetic peripheral neuropathy (DPN) in the US. Engensis (VM202) is a plasmid DNA designed to simultaneously express two isoforms of hepatocyte growth factor (HGF), HGF 728 and HGF 723. In addition to DPN, Engensis is also being studied in diabetic foot ulcers (DFU), amyotrophic lateral sclerosis (ALS), coronary artery disease (CAD), claudication, and Charcot-Marie-Tooth disease (CMT).
Ozanezumab is a monoclonal antibody designed for the treatment of ALS and multiple sclerosis.

Abacavir/dolutegravir/lamivudine, sold under the brand name Triumeq among others, is a fixed-dose combination antiretroviral medication for the treatment of HIV/AIDS. It is a combination of three medications with different and complementary mechanisms of action: abacavir, dolutegravir and lamivudine.
Genervon Biopharmaceuticals is a pharmaceutical company based in Pasadena, CA, focused on creating drugs for diseases of the central human nervous system. It is best known for its experimental drug, GM604, which seeks to treat Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease.
Research on amyotrophic lateral sclerosis (ALS) has focused on animal models of the disease, its mechanisms, ways to diagnose and track it, and treatments.
Merit Cudkowicz is an American neurologist and neuroscientist who studies amyotrophic lateral sclerosis (ALS). Cudkowicz is Julieanne Dorn Professor of Neurology at Harvard Medical School, director of the ALS clinic and the Neurological Clinical Research Institute at Massachusetts General Hospital (MGH) and chair of the Department of Neurology at MGH. Cudkowicz has led several large scale collaborations and clinical trials to test novel treatments for ALS and as of 2020 researches ways to detect early biomarkers of ALS to improve diagnosis.
Sodium phenylbutyrate/ursodoxicoltaurine, also known as sodium phenylbutyrate/taurursodiol and sold under the brand name Albrioza among others, is a fixed-dose combination medication used for the treatment of amyotrophic lateral sclerosis (ALS). It contains sodium phenylbutyrate and ursodoxicoltaurine (taurursodiol).










