
Cetuximab, sold under the brand name Erbitux, is an epidermal growth factor receptor (EGFR) inhibitor medication used for the treatment of metastatic colorectal cancer and head and neck cancer. Cetuximab is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
Adecatumumab (MT201) is a recombinant human IgG1 monoclonal antibody which is used to target tumor cells. It binds to the epithelial cell adhesion molecule, with the intent to trigger antibody-dependent cellular cytotoxicity. It was developed by Micromet Inc, which was acquired by Amgen.

KRAS is a gene that provides instructions for making a protein called K-Ras, a part of the RAS/MAPK pathway. The protein relays signals from outside the cell to the cell's nucleus. These signals instruct the cell to grow and divide (proliferate) or to mature and take on specialized functions (differentiate). It is called KRAS because it was first identified as a viral oncogene in the KirstenRAt Sarcoma virus. The oncogene identified was derived from a cellular genome, so KRAS, when found in a cellular genome, is called a proto-oncogene.
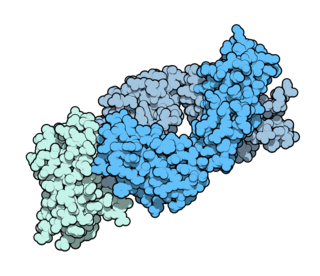
Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
Matuzumab is a humanized monoclonal antibody for the treatment of cancer. It binds to the epidermal growth factor receptor (EGFR) with high affinity. The mouse monoclonal antibody (mAb425) from which matuzumab was developed at the Wistar Institute in Philadelphia, Pennsylvania
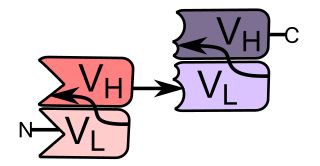
Bi-specific T-cell engagers (BiTEs) are a class of artificial bispecific monoclonal antibodies that are investigated for use as anti-cancer drugs. They direct a host's immune system, more specifically the T cells' cytotoxic activity, against cancer cells. BiTE is a registered trademark of Micromet AG.
Cixutumumab (IMC-A12) is a human monoclonal antibody for the treatment of solid tumors.
Ramucirumab is a fully human monoclonal antibody (IgG1) developed for the treatment of solid tumors. This drug was developed by ImClone Systems Inc. It was isolated from a native phage display library from Dyax.
Tigatuzumab (CS-1008) is a monoclonal antibody for the treatment of cancer. As of October 2009, a clinical trial for the treatment of pancreatic cancer, Phase II trials for colorectal cancer, non-small cell lung cancer, and ovarian cancer have been completed.
Glembatumumab vedotin is an antibody-drug conjugate (ADC) that targets cancer cells expressing transmembrane glycoprotein NMB (GPNMB).
Angiokinase inhibitors are a new therapeutic target for the management of cancer. They inhibit tumour angiogenesis, one of the key processes leading to invasion and metastasis of solid tumours, by targeting receptor tyrosine kinases. Examples include nintedanib, afatinib and motesanib.

Brigatinib, sold under the brand name Alunbrig among others, is a small-molecule targeted cancer therapy being developed by Ariad Pharmaceuticals, Inc. Brigatinib acts as both an anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitor.
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is administered by slow intravenous injection.

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.
MM-151 is an oligoclonal mixture of fully human monoclonal antibodies, which binds multiple parts of the EGFR molecule It has started clinical trials in patients with RAS wild-type colorectal cancers (CRCs) that were resistant to other anti-EGFR therapies. It is intended to overcome the problem of cancers becoming resistant to monoclonal antibody therapies.
Abituzumab is a humanized IgG2 monoclonal antibody (mAb) targeted at CD51 currently in development by Merck KGaA Darmstadt, Germany in an attempt to prevent bone lesion metastases in castration-resistant prostate cancer.
Amivantamab, sold under the brand name Rybrevant, is a bispecific monoclonal antibody used to treat non-small cell lung cancer. Amivantamab is a bispecific epidermal growth factor (EGF) receptor-directed and mesenchymal–epithelial transition (MET) receptor-directed antibody. It is the first treatment for adults with non-small cell lung cancer whose tumors have specific types of genetic mutations: epidermal growth factor receptor (EGFR) exon 20 insertion mutations.








