Bevacizumab, sold under the brand name Avastin among others, is a medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, glioblastoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).

Cetuximab, sold under the brand name Erbitux, is an epidermal growth factor receptor (EGFR) inhibitor medication used for the treatment of metastatic colorectal cancer and head and neck cancer. Cetuximab is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Radioimmunotherapy (RIT) uses an antibody labeled with a radionuclide to deliver cytotoxic radiation to a target cell. It is a form of unsealed source radiotherapy. In cancer therapy, an antibody with specificity for a tumor-associated antigen is used to deliver a lethal dose of radiation to the tumor cells. The ability for the antibody to specifically bind to a tumor-associated antigen increases the dose delivered to the tumor cells while decreasing the dose to normal tissues. By its nature, RIT requires a tumor cell to express an antigen that is unique to the neoplasm or is not accessible in normal cells.
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses.
Panitumumab, sold under the brand name Vectibix, is a fully human monoclonal antibody specific to the epidermal growth factor receptor.
Technetium (99mTc) sulesomab is a radio-pharmaceutical composed of anti-human mouse monoclonal antibody that targets the granulocyte associated NCA-90 cell antigen and a conjugated technetium-99m radionuclide. After intravenous administration, Leukoscan enables sensitive and specific whole body measurement of granulocyte infiltration and activation by gamma camera imaging of 99mTc-antibody bound cells. Total clearance of LeukoScan from blood samples after administration and imaging has been reported at 48 hour time points indicating limited retention of the agent in circulation

Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99, symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medical radioisotope in the world.
Technetium (99mTc) arcitumomab is a drug used for the diagnostic imaging of colorectal cancers, marketed by Immunomedics. It consists of the Fab' fragment of a monoclonal antibody and a radionuclide, technetium-99m.
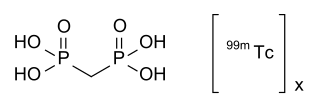
Technetium (99mTc) medronic acid is a pharmaceutical product used in nuclear medicine to localize bone metastases as well as other diseases that can alter the natural turn-over in the bone by bone scintigraphy.
Tigatuzumab (CS-1008) is a monoclonal antibody for the treatment of cancer. As of October 2009, a clinical trial for the treatment of pancreatic cancer, Phase II trials for colorectal cancer, non-small cell lung cancer, and ovarian cancer have been completed.
Brentuximab vedotin, sold under the brand name Adcetris, is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL), a type of T cell non-Hodgkin lymphoma. It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL. The drug is being jointly marketed by Millennium Pharmaceuticals outside the US and by Seattle Genetics in the US.
Olaratumab, sold under the brand name Lartruvo, is a monoclonal antibody medication developed by Eli Lilly and Company for the treatment of solid tumors. It is directed against the platelet-derived growth factor receptor alpha.

Plus Therapeutics, Inc. is a clinical-stage pharmaceutical company developing innovative, targeted radiotherapeutics for adults and children with rare and difficult-to-treat cancers. The company is headquartered in Austin, Texas, United States.
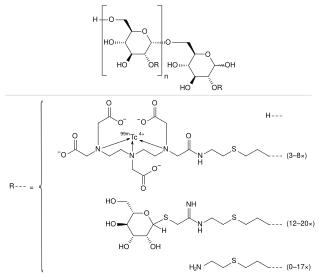
Technetium (99mTc) tilmanocept, trade name Lymphoseek, is a radiopharmaceutical diagnostic imaging agent used to locate lymph nodes which may be draining from tumors, and assist doctors in locating lymph nodes for removal during surgery.
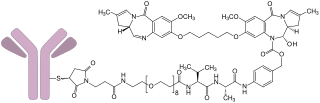
Loncastuximab tesirine, sold under the brand name Zynlonta, is a monoclonal antibody conjugate medication used to treat large B-cell lymphoma. It is an antibody-drug conjugate (ADC) composed of a humanized antibody targeting the protein CD19.
Dostarlimab, sold under the brand name Jemperli, is a monoclonal antibody used as a medication for the treatment of endometrial cancer. Dostarlimab is a programmed death receptor-1 (PD-1)–blocking monoclonal antibody.
Regdanvimab, sold under the brand name Regkirona, is a human monoclonal antibody used for the treatment of COVID-19. The antibody is directed against the spike protein of SARS-CoV-2. It is developed by Celltrion. The medicine is given by infusion (drip) into a vein.
Sotrovimab, sold under the brand name Xevudy, is a human neutralizing monoclonal antibody with activity against severe acute respiratory syndrome coronavirus 2, known as SARS-CoV-2. It was developed by GlaxoSmithKline and Vir Biotechnology, Inc. Sotrovimab is designed to attach to the spike protein of SARS-CoV-2.
Amivantamab, sold under the brand name Rybrevant, is a bispecific monoclonal antibody used to treat non-small cell lung cancer. Amivantamab is a bispecific epidermal growth factor (EGF) receptor-directed and mesenchymal–epithelial transition (MET) receptor-directed antibody. It is the first treatment for adults with non-small cell lung cancer whose tumors have specific types of genetic mutations: epidermal growth factor receptor (EGFR) exon 20 insertion mutations.







