
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.
Pittsburgh compound B (PiB) is a radioactive analog of thioflavin T, which can be used in positron emission tomography scans to image beta-amyloid plaques in neuronal tissue. Due to this property, Pittsburgh compound B may be used in investigational studies of Alzheimer's disease.

Tauopathies are a class of neurodegenerative diseases characterized by the aggregation of abnormal tau protein. Hyperphosphorylation of tau proteins causes them to dissociate from microtubules and form insoluble aggregates called neurofibrillary tangles. Various neuropathologic phenotypes have been described based on the anatomical regions and cell types involved as well as the unique tau isoforms making up these deposits. The designation 'primary tauopathy' is assigned to disorders where the predominant feature is the deposition of tau protein. Alternatively, diseases exhibiting tau pathologies attributed to different and varied underlying causes are termed 'secondary tauopathies'. Some neuropathologic phenotypes involving tau protein is Alzheimer's disease, Pick disease, Progressive supranuclear palsy, and corticobasal degeneration.
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET).

Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens, and is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems with language, disorientation, mood swings, loss of motivation, self-neglect, and behavioral issues. As a person's condition declines, they often withdraw from family and society. Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression can vary, the average life expectancy following diagnosis is three to twelve years.
Avid Radiopharmaceuticals is an American company, founded by Dr. Daniel Skovronsky, and based at the University City Science Center research campus in Philadelphia, Pennsylvania. The company has developed a radioactive tracer called florbetapir (18F). Florbetapir can be used to detect beta amyloid plaques in patients with memory problems using positron emission tomography (PET) scans, making the company the first to bring to market an FDA-approved method that can directly detect this hallmark pathology of Alzheimer's disease.
Alzheimer's Disease Neuroimaging Initiative (ADNI) is a multisite study that aims to improve clinical trials for the prevention and treatment of Alzheimer's disease (AD). This cooperative study combines expertise and funding from the private and public sector to study subjects with AD, as well as those who may develop AD and controls with no signs of cognitive impairment. Researchers at 63 sites in the US and Canada track the progression of AD in the human brain with neuroimaging, biochemical, and genetic biological markers. This knowledge helps to find better clinical trials for the prevention and treatment of AD. ADNI has made a global impact, firstly by developing a set of standardized protocols to allow the comparison of results from multiple centers, and secondly by its data-sharing policy which makes available all at the data without embargo to qualified researchers worldwide. To date, over 1000 scientific publications have used ADNI data. A number of other initiatives related to AD and other diseases have been designed and implemented using ADNI as a model. ADNI has been running since 2004 and is currently funded until 2021.

Brain positron emission tomography is a form of positron emission tomography (PET) that is used to measure brain metabolism and the distribution of exogenous radiolabeled chemical agents throughout the brain. PET measures emissions from radioactively labeled metabolically active chemicals that have been injected into the bloodstream. The emission data from brain PET are computer-processed to produce multi-dimensional images of the distribution of the chemicals throughout the brain.
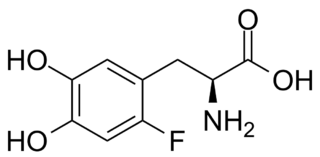
Fluorodopa, also known as FDOPA, is a fluorinated form of L-DOPA primarily synthesized as its fluorine-18 isotopologue for use as a radiotracer in positron emission tomography (PET).
Florbetapir (18F), sold under the brand name Amyvid, is a PET scanning radiopharmaceutical compound containing the radionuclide fluorine-18 that was approved for use in the United States in 2012, as a diagnostic tool for Alzheimer's disease. Florbetapir, like Pittsburgh compound B (PiB), binds to beta-amyloid, however fluorine-18 has a half-life of 109.75 minutes, in contrast to PiB's radioactive half life of 20 minutes. The longer life allows the tracer to accumulate significantly more in the brains of people with AD, particularly in the regions known to be associated with beta-amyloid deposits.
Florbetaben, sold under the brand name Neuraceq, is a diagnostic radiotracer developed for routine clinical application to visualize β-amyloid plaques in the brain. It is a fluorine-18 (18F)-labeled stilbene derivative.

Flutemetamol (18F) is a PET scanning radiopharmaceutical containing the radionuclide fluorine-18, used as a diagnostic tool for Alzheimer's disease.
Primary age-related tauopathy (PART) is a neuropathological designation introduced in 2014 to describe the neurofibrillary tangles (NFT) that are commonly observed in the brains of normally aged and cognitively impaired individuals that can occur independently of the amyloid plaques of Alzheimer's disease (AD). The term and diagnostic criteria for PART were developed by a large group of neuropathologists, spearheaded by Drs. John F. Crary and Peter T. Nelson. Despite some controversy, the term PART has been widely adopted, with the consensus criteria cited over 1130 times as of April 2023 according to Google Scholar.

Fluciclovine (18F), also known as anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, and sold under the brand name Axumin, is a diagnostic agent used for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated prostate specific antigen (PSA) levels.
Lecanemab, sold under the brand name Leqembi, is a monoclonal antibody medication used for the treatment of Alzheimer's disease. Lecanemab is an amyloid beta-directed antibody. It is given via intravenous infusion. The most common side effects of lecanemab include headache, infusion-related reactions, and amyloid-related imaging abnormalities, a side effect known to occur with the class of antibodies targeting amyloid.

Julie C. Price is an American medical physicist and professor of radiology at Massachusetts General Hospital (MGH), Harvard Medical School (HMS), as well as the director of PET Pharmacokinetic Modeling at the Athinoula A. Martinos Center at MGH. Price is a leader in the study and application of quantitative positron emission tomography (PET) methods. Prior to this, Price worked with Pittsburgh colleagues to lead the first fully quantitative pharmacokinetic evaluations of 11C-labeled Pittsburgh compound-B (PIB), one of the most widely used PET ligands for imaging amyloid beta plaques. As a principal investigator at MGH, Price continues work to validate novel PET methods for imaging biological markers of health and disease in studies of aging and neurodegeneration, including studies of glucose metabolism, protein expression, neurotransmitter system function, and tau and amyloid beta plaque burden.

Piflufolastat F-18, sold under the brand name Pylarify among others, is a radioactive diagnostic agent used for positron emission tomography (PET) imaging. It is given by intravenous injection.
Hartmuth Christian Kolb is a German chemist. He is considered one of the founders of click chemistry.
Neil Vasdev is a Canadian and American radiochemist and expert in nuclear medicine and molecular imaging, particularly in the application of PET. Radiotracers developed by the Vasdev Lab are in preclinical use worldwide, and many have been translated for first-in-human neuroimaging studies. He is the director and chief radiochemist of the Brain Health Imaging Centre and director of the Azrieli Centre for Neuro-Radiochemistry at the Centre for Addiction and Mental Health (CAMH). He is the Tier 1 Canada Research Chair in Radiochemistry and Nuclear Medicine, the endowed Azrieli Chair in Brain and Behaviour and Professor of Psychiatry at the University of Toronto. Vasdev has been featured on Global News, CTV, CNN, New York Times, Toronto Star and the Globe and Mail for his innovative research program.
Alzheimer's disease (AD) in African Americans is becoming a rising topic of interest in AD care, support, and scientific research, as African Americans are disproportionately affected by AD. Recent research on AD has shown that there are clear disparities in the disease among racial groups, with higher prevalence and incidence in African Americans than the overall average. Pathologies for Alzheimer’s also seem to manifest differently in African Americans, including with neuroinflammation markers, cognitive decline, and biomarkers. Although there are genetic risk factors for Alzheimer’s, these account for few cases in all racial groups.









