
Single-photon emission computed tomography is a nuclear medicine tomographic imaging technique using gamma rays. It is very similar to conventional nuclear medicine planar imaging using a gamma camera, but is able to provide true 3D information. This information is typically presented as cross-sectional slices through the patient, but can be freely reformatted or manipulated as required.

Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emitted from within the body rather than radiation that is transmitted through the body from external sources like X-ray generators. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine.

Technetium (99mTc) sestamibi (INN) is a pharmaceutical agent used in nuclear medicine imaging. The drug is a coordination complex consisting of the radioisotope technetium-99m bound to six (sesta=6) methoxyisobutylisonitrile (MIBI) ligands. The anion is not defined. The generic drug became available late September 2008. A scan of a patient using MIBI is commonly known as a "MIBI scan".

Scintigraphy, also known as a gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals) are taken internally and the emitted gamma radiation is captured by gamma cameras, which are external detectors that form two-dimensional images in a process similar to the capture of x-ray images. In contrast, SPECT and positron emission tomography (PET) form 3-dimensional images and are therefore classified as separate techniques from scintigraphy, although they also use gamma cameras to detect internal radiation. Scintigraphy is unlike a diagnostic X-ray where external radiation is passed through the body to form an image.
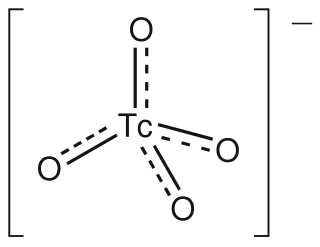
The pertechnetate ion is an oxyanion with the chemical formula TcO−
4. It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the 99mTc isotope which is commonly used in nuclear medicine in several nuclear scanning procedures.

A bone scan or bone scintigraphy is a nuclear medicine imaging technique of the bone. It can help diagnose a number of bone conditions, including cancer of the bone or metastasis, location of bone inflammation and fractures, and bone infection (osteomyelitis).

Sodium pertechnetate is the inorganic compound with the formula NaTcO4. This colourless salt contains the pertechnetate anion, TcO−
4 that has slightly distorted tetrahedron symmetry both at 296 K and at 100 K while the coordination polyhedron of the sodium cation is different from typical for scheelite structure. The radioactive 99m
Tc
O−
4 anion is an important radiopharmaceutical for diagnostic use. The advantages to 99m
Tc
include its short half-life of 6 hours and the low radiation exposure to the patient, which allow a patient to be injected with activities of more than 30 millicuries. Na[99m
Tc
O
4] is a precursor to a variety of derivatives that are used to image different parts of the body.
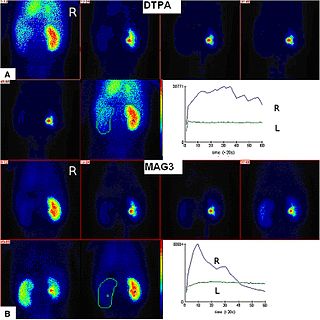
Radioisotope renography is a form of medical imaging of the kidneys that uses radiolabelling. A renogram, which may also be known as a MAG3 scan, allows a nuclear medicine physician or a radiologist to visualize the kidneys and learn more about how they are functioning. MAG3 is an acronym for mercapto acetyl tri glycine, a compound that is chelated with a radioactive element – technetium-99m.

A ventilation/perfusion lung scan, also called a V/Q lung scan, or ventilation/perfusion scintigraphy, is a type of medical imaging using scintigraphy and medical isotopes to evaluate the circulation of air and blood within a patient's lungs, in order to determine the ventilation/perfusion ratio. The ventilation part of the test looks at the ability of air to reach all parts of the lungs, while the perfusion part evaluates how well blood circulates within the lungs. As Q in physiology is the letter used to describe bloodflow the term V/Q scan emerged.

Nuclear pharmacy, also known as radiopharmacy, involves preparation of radioactive materials for patient administration that will be used to diagnose and treat specific diseases in nuclear medicine. It generally involves the practice of combining a radionuclide tracer with a pharmaceutical component that determines the biological localization in the patient. Radiopharmaceuticals are generally not designed to have a therapeutic effect themselves, but there is a risk to staff from radiation exposure and to patients from possible contamination in production. Due to these intersecting risks, nuclear pharmacy is a heavily regulated field. The majority of diagnostic nuclear medicine investigations are performed using technetium-99m.

Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99, symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medical radioisotope in the world.

Myocardial perfusion imaging or scanning is a nuclear medicine procedure that illustrates the function of the heart muscle (myocardium).
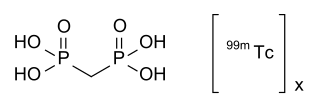
Technetium (99mTc) medronic acid is a pharmaceutical product used in nuclear medicine to localize bone metastases as well as other diseases that can alter the natural turn-over in the bone by bone scintigraphy.

Cholescintigraphy or hepatobiliary scintigraphy is scintigraphy of the hepatobiliary tract, including the gallbladder and bile ducts. The image produced by this type of medical imaging, called a cholescintigram, is also known by other names depending on which radiotracer is used, such as HIDA scan, PIPIDA scan, DISIDA scan, or BrIDA scan. Cholescintigraphic scanning is a nuclear medicine procedure to evaluate the health and function of the gallbladder and biliary system. A radioactive tracer is injected through any accessible vein and then allowed to circulate to the liver, where it is excreted into the bile ducts and stored by the gallbladder until released into the duodenum.

An octreotide scan is a type of SPECT scintigraphy used to find carcinoid, pancreatic neuroendocrine tumors, and to localize sarcoidosis. It is also called somatostatin receptor scintigraphy (SRS). Octreotide, a drug similar to somatostatin, is radiolabeled with indium-111, and is injected into a vein and travels through the bloodstream. The radioactive octreotide attaches to tumor cells that have receptors for somatostatin. A gamma camera detects the radioactive octreotide, and makes pictures showing where the tumor cells are in the body, typically by a SPECT technique. A technetium-99m based radiopharmaceutical kit is also available.
Perfusion is the passage of fluid through the lymphatic system or blood vessels to an organ or a tissue. The practice of perfusion scanning is the process by which this perfusion can be observed, recorded and quantified. The term perfusion scanning encompasses a wide range of medical imaging modalities.

Technetium (99mTc) exametazime is a radiopharmaceutical sold under the trade name Ceretec, and is used by nuclear medicine physicians for the detection of altered regional cerebral perfusion in stroke and other cerebrovascular diseases. It can also be used for the labelling of leukocytes to localise intra-abdominal infections and inflammatory bowel disease. Exametazime is sometimes referred to as hexamethylpropylene amine oxime or HMPAO, although correct chemical names are:

Technetium (99mTc) tetrofosmin is a drug used in nuclear medicine cardiac imaging. It is sold under the brand name Myoview. The radioisotope, technetium-99m, is chelated by two 1,2-bis[di-(2-ethoxyethyl)phosphino]ethane ligands which belong to the group of diphosphines and which are referred to as tetrofosmin.

Technetium (99mTc) mebrofenin is a diagnostic radiopharmaceutical used for imaging of the liver and the gallbladder. Under the brand name Choletec it is available from Bracco Diagnostic. Supplied as a sterile kit of mebrofenin and dehydrated stannous fluoride. The vial is reconstituted with 1 to 5 mL up to 3.7 gigabecquerels (100 mCi) of sodium pertechnetate solution to form the final radio labeled 99mTc mebrofenin.
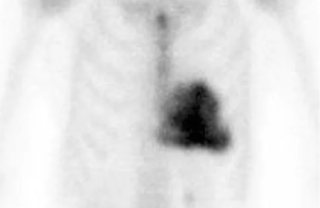
A DPD scan is a type of nuclear medicine imaging test which uses radioactive technetium-99m (99mTc) and 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) to diagnose cardiac amyloidosis. The radiopharmaceutical is taken up only in patients with ATTR amyloidosis, making it a useful tool to differentiate from AL amyloidosis.



















