
Osteoporosis is a systemic skeletal disorder characterized by low bone mass, micro-architectural deterioration of bone tissue leading to more porous bone, and consequent increase in fracture risk. It is the most common reason for a broken bone among the elderly. Bones that commonly break include the vertebrae in the spine, the bones of the forearm, the wrist, and the hip. Until a broken bone occurs there are typically no symptoms. Bones may weaken to such a degree that a break may occur with minor stress or spontaneously. After the broken bone heals, the person may have chronic pain and a decreased ability to carry out normal activities.

Parathyroid hormone (PTH), also called parathormone or parathyrin, is a peptide hormone secreted by the parathyroid glands that regulates the serum calcium concentration through its effects on bone, kidney, and intestine.

Bisphosphonates are a class of drugs that prevent the loss of bone density, used to treat osteoporosis and similar diseases. They are the most commonly prescribed drugs used to treat osteoporosis. They are called bisphosphonates because they have two phosphonate groups. They are thus also called diphosphonates.
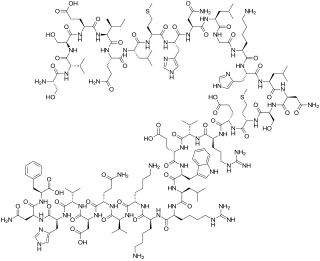
Teriparatide, sold under the brand name Forteo, is a form of parathyroid hormone (PTH) consisting of the first (N-terminus) 34 amino acids, which is the bioactive portion of the hormone. It is an effective anabolic agent used in the treatment of some forms of osteoporosis. Teriparatide is a recombinant human parathyroid hormone analog. It has an identical sequence to the 34 N-terminal amino acids of the 84-amino acid human parathyroid hormone.

Amgen Inc. is an American multinational biopharmaceutical company headquartered in Thousand Oaks, California. One of the world's largest independent biotechnology companies, Amgen's Thousand Oaks staff in 2022 numbered approximately 5,000 and included hundreds of scientists, making Amgen the largest employer in Ventura County. As of 2022, Amgen has approximately 24,000 staff in total.

Alendronic acid, sold under the brand name Fosamax among others, is a bisphosphonate medication used to treat osteoporosis and Paget's disease of bone. It is taken by mouth. Use is often recommended together with vitamin D, calcium supplementation, and lifestyle changes.
Adalimumab, sold under the brand name Humira and others, is a disease-modifying antirheumatic drug and monoclonal antibody used to treat rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, and uveitis. It is administered by subcutaneous injection. It works by inactivating tumor necrosis factor-alpha (TNFα).
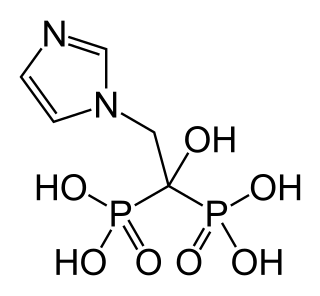
Zoledronic acid, also known as zoledronate and sold under the brand name Zometa by Novartis among others, is a medication used to treat a number of bone diseases. These include osteoporosis, high blood calcium due to cancer, bone breakdown due to cancer, Paget's disease of bone and Duchenne muscular dystrophy (DMD). It is given by injection into a vein.

Bazedoxifene, used as bazedoxifene acetate, is a medication for bone problems and possibly for cancer. It is a third-generation selective estrogen receptor modulator (SERM). Since late 2013 it has had U.S. FDA approval for bazedoxifene as part of the combination drug Duavee in the prevention of postmenopausal osteoporosis. It is also being studied for possible treatment of breast cancer and pancreatic cancer.
Ranibizumab, sold under the brand name Lucentis among others, is a monoclonal antibody fragment (Fab) created from the same parent mouse antibody as bevacizumab. It is an anti-angiogenic that is approved to treat the "wet" type of age-related macular degeneration, diabetic retinopathy, and macular edema due to branch retinal vein occlusion or central retinal vein occlusion.
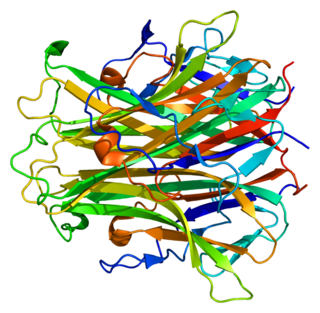
Receptor activator of nuclear factor kappa-Β ligand (RANKL), also known as tumor necrosis factor ligand superfamily member 11 (TNFSF11), TNF-related activation-induced cytokine (TRANCE), osteoprotegerin ligand (OPGL), and osteoclast differentiation factor (ODF), is a protein that in humans is encoded by the TNFSF11 gene.
Eculizumab, sold under the brand name Soliris among others, is a recombinant humanized monoclonal antibody used to treat paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), generalized myasthenia gravis, and neuromyelitis optica. In people with PNH, it reduces both the destruction of red blood cells and need for blood transfusion, but does not appear to affect the risk of death. Eculizumab was the first drug approved for each of its uses, and its approval was granted based on small trials. It is given by intravenous infusion.

Strontium ranelate, a strontium(II) salt of ranelic acid, is a medication for osteoporosis marketed as Protelos or Protos by Servier. Studies indicate it can also slow the course of osteoarthritis of the knee. The drug is unusual in that it both increases deposition of new bone by osteoblasts and reduces the resorption of bone by osteoclasts. It is therefore promoted as a "dual action bone agent" (DABA).
A biosimilar is a biologic medical product that is almost an identical copy of an original product that is manufactured by a different company. Biosimilars are officially approved versions of original "innovator" products and can be manufactured when the original product's patent expires. Reference to the innovator product is an integral component of the approval.
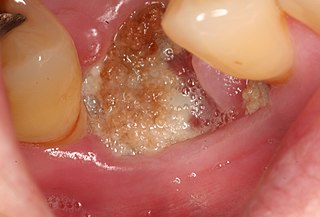
Medication-related osteonecrosis of the jaw is progressive death of the jawbone in a person exposed to a medication known to increase the risk of disease, in the absence of a previous radiation treatment. It may lead to surgical complication in the form of impaired wound healing following oral and maxillofacial surgery, periodontal surgery, or endodontic therapy.
Romosozumab, sold under the brand name Evenity, is a medication used to treat osteoporosis. It has been found to decrease the risk of fractures of the spine.
Abaloparatide, sold under the brand name Tymlos among others, is a parathyroid hormone-related protein (PTHrP) analog medication used to treat osteoporosis. It is an anabolic agent.
Apremilast, sold under the brand name Otezla among others, is a medication for the treatment of certain types of psoriasis and psoriatic arthritis. The drug acts as a selective inhibitor of the enzyme phosphodiesterase 4 (PDE4) and inhibits spontaneous production of TNF-alpha from human rheumatoid synovial cells. It is taken by mouth.
Eldecalcitol is an analog of calcitriol, the active form of vitamin D.
Recombinant human parathyroid hormone, sold under the brand name Preotact among others, is an artificially manufactured form of the parathyroid hormone used to treat hypoparathyroidism. Recombinant human parathyroid hormone is used in the treatment of osteoporosis in postmenopausal women at high risk of osteoporotic fractures. A significant reduction in the incidence of vertebral fractures has been demonstrated. It is used in combination with calcium and vitamin D supplements.













