The thrombopoietin receptor also known as the myeloproliferative leukemia protein or CD110 (Cluster of Differentiation 110) is a protein that in humans is encoded by the MPL (myeloproliferative leukemia virus) oncogene. [5]
The thrombopoietin receptor also known as the myeloproliferative leukemia protein or CD110 (Cluster of Differentiation 110) is a protein that in humans is encoded by the MPL (myeloproliferative leukemia virus) oncogene. [5]
In 1990 an oncogene, v-mpl, was identified from the murine myeloproliferative leukemia virus that was capable of immortalizing bone marrow hematopoietic cells from different lineages. In 1992 the human homologue, named, c-mpl, was cloned. Sequence data revealed that c-mpl encoded a protein that was homologous with members of the hematopoietic receptor superfamily. Presence of anti-sense oligodeoxynucleotides of c-mpl inhibited megakaryocyte colony formation.
The ligand for c-mpl, thrombopoietin, was cloned in 1994. Thrombopoietin was shown to be the major regulator of megakaryocytopoiesis and platelet formation.
The protein encoded by the c-mpl gene, CD110, is a 635 amino acid transmembrane domain, with two extracellular cytokine receptor domains and two intracellular cytokine receptor box motifs . TPO-R deficient mice were severely thrombocytopenic, emphasizing the important role of CD110 and thrombopoietin in megakaryocyte and platelet formation. Upon binding of thrombopoietin, CD110 is dimerized and the JAK family of non-receptor tyrosine kinases, as well as the STAT family, the MAPK family, the adaptor protein Shc and the receptors themselves become tyrosine phosphorylated. [5]
Myeloproliferative leukemia virus oncogene has been shown to interact with:
Inactivating mutations in this gene have been shown to cause familial aplastic anemia. [9]
Specific mutations to this gene are associated with myelofibrosis and essential thrombocythemia. [10] In essential thrombocythemia, mutations occur at position 505 or 515 in the protein. In myelofibrosis, a mutation occurs at position 515. These mutations lead to the production of thrombopoietin receptors that are permanently activated, which results in the overproduction of abnormal megakaryocytes. [11]
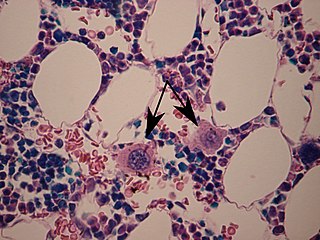
A megakaryocyte is a large bone marrow cell with a lobated nucleus responsible for the production of blood thrombocytes (platelets), which are necessary for normal blood clotting. In humans, megakaryocytes usually account for 1 out of 10,000 bone marrow cells, but can increase in number nearly 10-fold during the course of certain diseases. Owing to variations in combining forms and spelling, synonyms include megalokaryocyte and megacaryocyte.

Thrombopoietin (THPO) also known as megakaryocyte growth and development factor (MGDF) is a protein that in humans is encoded by the THPO gene.

The Philadelphia chromosome or Philadelphia translocation (Ph) is a specific genetic abnormality in chromosome 22 of leukemia cancer cells. This chromosome is defective and unusually short because of reciprocal translocation, t(9;22)(q34;q11), of genetic material between chromosome 9 and chromosome 22, and contains a fusion gene called BCR-ABL1. This gene is the ABL1 gene of chromosome 9 juxtaposed onto the breakpoint cluster region BCR gene of chromosome 22, coding for a hybrid protein: a tyrosine kinase signaling protein that is "always on", causing the cell to divide uncontrollably by interrupting the stability of the genome and impairing various signaling pathways governing the cell cycle.
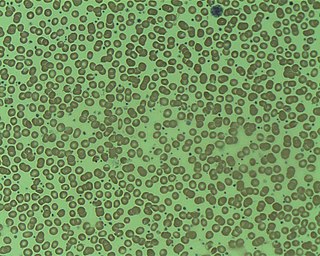
Essential thrombocythemia (ET) is a rare chronic blood cancer characterised by the overproduction of platelets (thrombocytes) by megakaryocytes in the bone marrow. It may, albeit rarely, develop into acute myeloid leukemia or myelofibrosis. It is a type of myeloproliferative neoplasm wherein the body makes too many white or red blood cells, or platelets.
The JAK-STAT signaling pathway is a chain of interactions between proteins in a cell, and is involved in processes such as immunity, cell division, cell death, and tumour formation. The pathway communicates information from chemical signals outside of a cell to the cell nucleus, resulting in the activation of genes through the process of transcription. There are three key parts of JAK-STAT signalling: Janus kinases (JAKs), signal transducer and activator of transcription proteins (STATs), and receptors. Disrupted JAK-STAT signalling may lead to a variety of diseases, such as skin conditions, cancers, and disorders affecting the immune system.
Primary myelofibrosis (PMF) is a rare bone marrow blood cancer. It is classified by the World Health Organization (WHO) as a type of myeloproliferative neoplasm, a group of cancers in which there is growth of abnormal cells in the bone marrow. This is most often associated with a somatic mutation in the JAK2, CALR, or MPL gene markers. In PMF, the healthy marrow is replaced by scar tissue (fibrosis), resulting in a lack of production of normal blood cells. Symptoms include anemia, increased infection and an enlarged spleen (splenomegaly).

Myeloproliferative neoplasms (MPNs) are a group of rare blood cancers in which excess red blood cells, white blood cells or platelets are produced in the bone marrow. Myelo refers to the bone marrow, proliferative describes the rapid growth of blood cells and neoplasm describes that growth as abnormal and uncontrolled.
Mean platelet volume (MPV) is a machine-calculated measurement of the average size of platelets found in blood and is typically included in blood tests as part of the CBC. Since the average platelet size is larger when the body is producing increased numbers of platelets, the MPV test results can be used to make inferences about platelet production in bone marrow or platelet destruction problems.

GATA-binding factor 1 or GATA-1 is the founding member of the GATA family of transcription factors. This protein is widely expressed throughout vertebrate species. In humans and mice, it is encoded by the GATA1 and Gata1 genes, respectively. These genes are located on the X chromosome in both species.
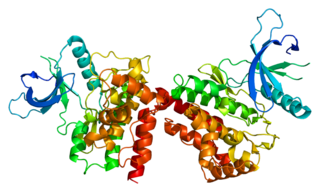
Janus kinase 2 is a non-receptor tyrosine kinase. It is a member of the Janus kinase family and has been implicated in signaling by members of the type II cytokine receptor family, the GM-CSF receptor family, the gp130 receptor family, and the single chain receptors.

ETV6 protein is a transcription factor that in humans is encoded by the ETV6 gene. The ETV6 protein regulates the development and growth of diverse cell types, particularly those of hematological tissues. However, its gene, ETV6 frequently suffers various mutations that lead to an array of potentially lethal cancers, i.e., ETV6 is a clinically significant proto-oncogene in that it can fuse with other genes to drive the development and/or progression of certain cancers. However, ETV6 is also an anti-oncogene or tumor suppressor gene in that mutations in it that encode for a truncated and therefore inactive protein are also associated with certain types of cancers.

Tyrosine-protein kinase ABL2 also known as Abelson-related gene (Arg) is an enzyme that in humans is encoded by the ABL2 gene.

SH2B adapter protein 3 (SH2B3), also known as lymphocyte adapter protein (LNK), is a protein that in humans is encoded by the SH2B3 gene on chromosome 12.

MASTL is an official symbol provided by HGNC for human gene whose official name is micro tubule associated serine/threonine kinase like. This gene is 32,1 kbps long. This gene is also known as GW, GWL, THC2, MAST-L, GREATWALL. This is present in mainly mammalian cells like human, house mouse, cattle, monkey, etc. It is in the 10th chromosome of the mammalian nucleus. Recent studies have been carried on zebrafish and frogs. This gene encodes for the protein micro tubule associated serine/threonine kinase and its sub-classes.

Acute megakaryoblastic leukemia (AMKL) is life-threatening leukemia in which malignant megakaryoblasts proliferate abnormally and injure various tissues. Megakaryoblasts are the most immature precursor cells in a platelet-forming lineage; they mature to promegakaryocytes and, ultimately, megakaryocytes which cells shed membrane-enclosed particles, i.e. platelets, into the circulation. Platelets are critical for the normal clotting of blood. While malignant megakaryoblasts usually are the predominant proliferating and tissue-damaging cells, their similarly malignant descendants, promegakaryocytes and megakaryocytes, are variable contributors to the malignancy.

Congenital amegakaryocytic thrombocytopenia (CAMT) is a rare inherited disorder.
Kenneth Kaushansky, M.D., Master of the American College of Physicians (MACP) is an American medical doctor, hematologist, former editor of the medical journal Blood, and has served as the Dean of the Stony Brook University School of Medicine since July 2010. Prior to moving to Stony Brook, he was the Helen M. Ranney Professor, and Chair of the Department of Medicine at University of California, San Diego School of Medicine.
Clonal hypereosinophilia, also termed primary hypereosinophilia or clonal eosinophilia, is a grouping of hematological disorders all of which are characterized by the development and growth of a pre-malignant or malignant population of eosinophils, a type of white blood cell that occupies the bone marrow, blood, and other tissues. This population consists of a clone of eosinophils, i.e. a group of genetically identical eosinophils derived from a sufficiently mutated ancestor cell.

William Vainchenker, born on 16 December 1947, is a French medical doctor and researcher. He is considered a specialist in hematopoiesis.
Transient myeloproliferative disease (TMD) occurs in a significant percentage of individuals born with the congenital genetic disorder, Down syndrome. It may occur in individuals who are not diagnosed with the syndrome but have some hematological cells containing genetic abnormalities that are similar to those found in Down syndrome. TMD usually develops in utero, is diagnosed prenatally or within ~3 months of birth, and thereafter resolves rapidly and spontaneously. However, during the prenatal-to-postnatal period, the disease may cause irreparable damage to various organs and in ~20% of individuals death. Moreover, ~10% of individuals diagnosed with TMD develop acute megakaryoblastic leukemia at some time during the 5 years following its resolution. TMD is a life-threatening, precancerous condition in fetuses as well as infants in their first few months of life.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.