Aplastic anemia (AA) is a severe hematologic condition in which the body fails to make blood cells in sufficient numbers. Aplastic anemia is associated with cancer and various cancer syndromes. Blood cells are produced in the bone marrow by stem cells that reside there. Aplastic anemia causes a deficiency of all blood cell types: red blood cells, white blood cells, and platelets.
Hematopoietic stem-cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood in order to replicate inside of a patient and to produce additional normal blood cells. It may be autologous, allogeneic or syngeneic.

Graft-versus-host disease (GvHD) is a syndrome, characterized by inflammation in different organs. GvHD is commonly associated with bone marrow transplants and stem cell transplants.

Hematopoietic stem cells (HSCs) are the stem cells that give rise to other blood cells. This process is called haematopoiesis. In vertebrates, the very first definitive HSCs arise from the ventral endothelial wall of the embryonic aorta within the (midgestational) aorta-gonad-mesonephros region, through a process known as endothelial-to-hematopoietic transition. In adults, haematopoiesis occurs in the red bone marrow, in the core of most bones. The red bone marrow is derived from the layer of the embryo called the mesoderm.

Interleukin 3 (IL-3) is a protein that in humans is encoded by the IL3 gene localized on chromosome 5q31.1. Sometimes also called colony-stimulating factor, multi-CSF, mast cell growth factor, MULTI-CSF, MCGF; MGC79398, MGC79399: the protein contains 152 amino acids and its molecular weight is 17 kDa. IL-3 is produced as a monomer by activated T cells, monocytes/macrophages and stroma cells. The major function of IL-3 cytokine is to regulate the concentrations of various blood-cell types. It induces proliferation and differentiation in both early pluripotent stem cells and committed progenitors. It also has many more specific effects like the regeneration of platelets and potentially aids in early antibody isotype switching.
Cord blood is blood that remains in the placenta and in the attached umbilical cord after childbirth. Cord blood is collected because it contains stem cells, which can be used to treat hematopoietic and genetic disorders such as cancer. There is growing interest from cell therapeutics companies in developing genetically modified allogenic natural killer cells from umbilical cord blood as an alternative to CAR T cell therapies for rare diseases.

Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells, they can be found in juvenile, adult animals, and humans, unlike embryonic stem cells.

Extramedullary hematopoiesis refers to hematopoiesis occurring outside of the medulla of the bone. It can be physiologic or pathologic.
Hemangioblasts are the multipotent precursor cells that can differentiate into both hematopoietic and endothelial cells. In the mouse embryo, the emergence of blood islands in the yolk sac at embryonic day 7 marks the onset of hematopoiesis. From these blood islands, the hematopoietic cells and vasculature are formed shortly after. Hemangioblasts are the progenitors that form the blood islands. To date, the hemangioblast has been identified in human, mouse and zebrafish embryos.

Sialomucin core protein 24 also known as endolyn or CD164 is a protein that in humans is encoded by the CD164 gene. CD164 functions as a cell adhesion molecule.

Cluster of differentiation antigen 135 (CD135) also known as fms like tyrosine kinase 3, receptor-type tyrosine-protein kinase FLT3, or fetal liver kinase-2 (Flk2) is a protein that in humans is encoded by the FLT3 gene. FLT3 is a cytokine receptor which belongs to the receptor tyrosine kinase class III. CD135 is the receptor for the cytokine Flt3 ligand (FLT3L).

Fms-related tyrosine kinase 3 ligand (FLT3LG) is a protein which in humans is encoded by the FLT3LG gene.

CFU-GEMM is a colony forming unit that generates myeloid cells. CFU-GEMM cells are the oligopotential progenitor cells for myeloid cells; they are thus also called common myeloid progenitor cells or myeloid stem cells. "GEMM" stands for granulocyte, erythrocyte, monocyte, megakaryocyte.
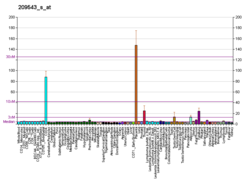
Stem cell markers are genes and their protein products used by scientists to isolate and identify stem cells. Stem cells can also be identified by functional assays. Below is a list of genes/protein products that can be used to identify various types of stem cells, or functional assays that do the same. The initial version of the list below was obtained by mining the PubMed database as described in
KSL cells in cell biology are an early form of mouse/murine hematopoietic stem cells. Characteristics are Kit (+), Sca-1 (+) and Lin (-). HSCs [Hematopoietic stem cells] in murine cultures show phenotypic markers as being CD34-, CD150+, and Flt3- for LTR [long-term reconstitution]. These phenotypic markers are used when purifying hematopoietic stem cells from the many other differentiated cells in the bone marrow.

Mesenchymal stem cells (MSCs) also known as mesenchymal stromal cells or medicinal signaling cells are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, myocytes and adipocytes.
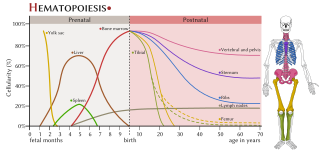
The haematopoietic system is the system in the body involved in the creation of the cells of blood.
Many human blood cells, such as red blood cells (RBCs), immune cells, and even platelets all originate from the same progenitor cell, the hematopoietic stem cell (HSC). As these cells are short-lived, there needs to be a steady turnover of new blood cells and the maintenance of an HSC pool. This is broadly termed hematopoiesis. This event requires a special environment, termed the hematopoietic stem cell niche, which provides the protection and signals necessary to carry out the differentiation of cells from HSC progenitors. This niche relocates from the yolk sac to eventually rest in the bone marrow of mammals. Many pathological states can arise from disturbances in this niche environment, highlighting its importance in maintaining hematopoiesis.
In the immune system, veto cells are white blood cells that have a selective immunomodulation properties. Veto cells were first described in 1979 as cells that “can prevent generation of cytotoxic lymphocytes by normal spleen cells against self-antigens”. Hence, veto cells delete T cells that recognize the veto cells.
Since haematopoietic stem cells cannot be isolated as a pure population, it is not possible to identify them under a microscope. Therefore, there are many techniques to isolate haematopoietic stem cells (HSCs). HSCs can be identified or isolated by the use of flow cytometry where the combination of several different cell surface markers is used to separate the rare HSCs from the surrounding blood cells. HSCs lack expression of mature blood cell markers and are thus, called Lin-. Lack of expression of lineage markers is used in combination with detection of several positive cell-surface markers to isolate HSCs. In addition, HSCs are characterized by their small size and low staining with vital dyes such as rhodamine 123 or Hoechst 33342.