
Leptin is a protein hormone predominantly made by adipocytes. Its primary role is likely to regulate long-term energy balance.

Adipose tissue is a loose connective tissue composed mostly of adipocytes. It also contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and a variety of immune cells such as adipose tissue macrophages. Its main role is to store energy in the form of lipids, although it also cushions and insulates the body.
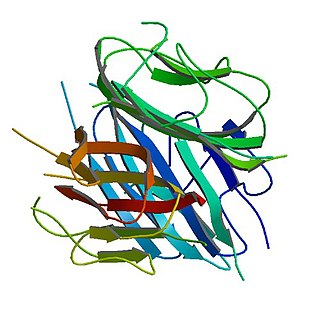
Adiponectin is a protein hormone and adipokine, which is involved in regulating glucose levels and fatty acid breakdown. In humans, it is encoded by the ADIPOQ gene and is produced primarily in adipose tissue, but also in muscle and even in the brain.

Resistin also known as adipose tissue-specific secretory factor (ADSF) or C/EBP-epsilon-regulated myeloid-specific secreted cysteine-rich protein (XCP1) is a cysteine-rich peptide hormone derived from adipose tissue that in humans is encoded by the RETN gene.

Protein tyrosine phosphatase, receptor type, C also known as PTPRC is an enzyme that, in humans, is encoded by the PTPRC gene. PTPRC is also known as CD45 antigen, which was originally called leukocyte common antigen (LCA).

The prolactin-releasing peptide receptor (PrRPR) also known as G-protein coupled receptor 10 (GPR10) is a protein that in humans is encoded by the PRLHR gene.

Melanocortin 4 receptor (MC4R) is a melanocortin receptor that in humans is encoded by the MC4R gene. It encodes the MC4R protein, a G protein-coupled receptor (GPCR) that binds α-melanocyte stimulating hormone (α-MSH). In mouse models, MC4 receptors have been found to be involved in feeding behaviour, the regulation of metabolism, sexual behaviour, and male erectile function.

ADGRV1, also known as G protein-coupled receptor 98 (GPR98) or Very Large G-protein coupled receptor 1 (VLGR1), is a protein that in humans is encoded by the GPR98 gene. Several alternatively spliced transcripts have been described.

Melanocortin 3 receptor (MC3R) is a protein that in humans is encoded by the MC3R gene.

Interleukin 10 receptor, beta subunit is a subunit for the interleukin-10 receptor. IL10RB is its human gene.

Interleukin 11 receptor, alpha subunit is a subunit of the interleukin 11 receptor. IL11RA is its human gene.

Receptor-type tyrosine-protein phosphatase N2 (R-PTP-N2) also known as islet cell autoantigen-related protein (ICAAR) and phogrin is an enzyme that in humans is encoded by the PTPRN2 gene. PTPRN and PTPRN2 are both found to be major autoantigens associated with insulin-dependent diabetes mellitus.

The ob/ob or obese mouse is a mutant mouse that eats excessively due to mutations in the gene responsible for the production of leptin and becomes profoundly obese. It is an animal model of type II diabetes. Identification of the gene mutated in ob led to the discovery of the hormone leptin, which is important in the control of appetite.

The Interleukin-2 receptor alpha chain is a protein involved in the assembly of the high-affinity Interleukin-2 receptor, consisting of alpha (IL2RA), beta (IL2RB) and the common gamma chain (IL2RG). As the name indicates, this receptor interacts with Interleukin-2, a pleiotropic cytokine which plays an important role in immune homeostasis.
Adipose tissue is an endocrine organ that secretes numerous protein hormones, including leptin, adiponectin, and resistin. These hormones generally influence energy metabolism, which is of great interest to the understanding and treatment of type 2 diabetes and obesity.
RPGRIP1L is a human gene.
Douglas L. Coleman was a scientist and professor emeritus at the Jackson Laboratory, in Bar Harbor, Maine. His work predicted that there exists a hormone that can cause mice to feel full, and that a mutation in the gene encoding this hormone can lead to obesity. The gene and corresponding hormone were discovered about 20 years later by Jeffrey M. Friedman, Rudolph Leibel, and their research teams at Rockefeller University, which Friedman named leptin.

Teleost leptins are a family of peptide hormones found in fish (teleostei) that are orthologs of the mammalian hormone leptin. The teleost and mammalian leptins appear to have similar functions, namely, regulation of energy intake and expenditure.

Rudolph Leibel is the Christopher J. Murphy Professor of Diabetes Research, Professor of Pediatrics and Medicine at Columbia University Medical Center, and Director of the Division of Molecular Genetics in the Department of Pediatrics. He is also co-director of the Naomi Berrie Diabetes Center and executive director of the Russell and Angelica Berrie Program in Cellular Therapy, Co-director of the New York Obesity Research Center and the Columbia University Diabetes and Endocrinology Research Center.
Louis Anthony Tartaglia is an American biochemist, pharmaceutical scientist, and entrepreneur. As a scientist, he is known for first identifying and cloning the leptin receptor in 1995, a discovery that prompted immediate coverage in US national media given its expected clinical significance. He is also known for studying signaling mechanisms from the tumor necrosis factor (TNF) receptors, and for publishing studies in the fields of obesity and diabetes which are often discussed in subject reviews. After moving from academia to industry in 1990, for over a decade he accompanied the growth of Millennium Pharmaceuticals, reaching top positions within the company. From executive roles he has occupied in venture capital firms, and as a member of several advisory boards, Tartaglia has helped start a number of therapeutics oriented companies that have found their way into the market, among them Agios, Editas, Rhythm, and Zafgen.



















