Darbepoetin alfa (INN) is a re-engineered form of erythropoietin containing 5 amino acid changes resulting in the creation of 2 new sites for N-linked carbohydrate addition. It has a 3-fold longer serum half-life compared to epoetin alpha and epoetin beta. It stimulates erythropoiesis by the same mechanism as rHuEpo and is used to treat anemia, commonly associated with chronic kidney failure and cancer chemotherapy. Darbepoetin is marketed by Amgen under the trade name Aranesp.

Anemia or anaemia is a blood disorder in which the blood has a reduced ability to carry oxygen due to a lower than normal number of red blood cells, a reduction in the amount of hemoglobin or hemoglobin abnormalities. The name is derived from Ancient Greek: ἀναιμία anaimia, meaning 'lack of blood', from ἀν- an-, 'not' and αἷμα haima, 'blood'. When anemia comes on slowly, the symptoms are often vague, such as tiredness, weakness, shortness of breath, headaches, and a reduced ability to exercise. When anemia is acute, symptoms may include confusion, feeling like one is going to pass out, loss of consciousness, and increased thirst. Anemia must be significant before a person becomes noticeably pale. Symptoms of anemia depend on how quickly hemoglobin decreases. Additional symptoms may occur depending on the underlying cause. Preoperative anemia can increase the risk of needing a blood transfusion following surgery. Anemia can be temporary or long term and can range from mild to severe.
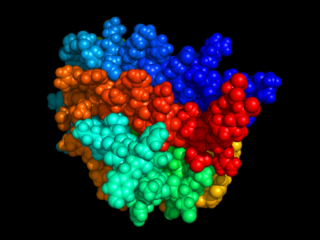
Erythropoietin, also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bone marrow. Low levels of EPO are constantly secreted in sufficient quantities to compensate for normal red blood cell turnover. Common causes of cellular hypoxia resulting in elevated levels of EPO include any anemia, and hypoxemia due to chronic lung disease.
ATC code B03Antianemic preparations is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup B03 is part of the anatomical group B Blood and blood forming organs.
Anemia of chronic disease (ACD) or anemia of chronic inflammation is a form of anemia seen in chronic infection, chronic immune activation, and malignancy. These conditions all produce elevation of interleukin-6, which stimulates hepcidin production and release from the liver. Hepcidin production and release shuts down ferroportin, a protein that controls export of iron from the gut and from iron storing cells. As a consequence, circulating iron levels are reduced. Other mechanisms may also play a role, such as reduced erythropoiesis. It is also known as anemia of inflammation, or anemia of inflammatory response.
Epoetin alfa is a human erythropoietin produced in cell culture using recombinant DNA technology. Authorised by the European Medicines Agency on 28 August 2007, it stimulates erythropoiesis and is used to treat anemia, commonly associated with chronic kidney failure and cancer chemotherapy.
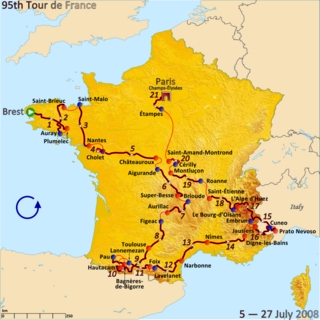
The 2008 Tour de France was the 95th running of the race. The event took place from 5 to 27 July. Starting in the French city of Brest, the tour entered Italy on the 15th stage and returned to France during the 16th, heading for Paris, its regular final destination, which was reached in the 21st stage. The race was won by Carlos Sastre.

Riccardo Riccò is an Italian professional road bicycle racer, who is suspended from all competition until 2024. He was previously ejected from the 2008 Tour de France for doping violations and suspended. Riccò returned to competition in late 2010, but in February 2011 he was fired by his team, Vacansoleil–DCM, after he became seriously ill allegedly through a self-administered autologous blood transfusion. He then signed to UCI Continental team Meridiana–Kamen.
The Festina affair was a series of doping scandals within the sport of professional cycling that occurred during and after the 1998 Tour de France. The affair began when a large haul of doping products was found in a support car belonging to the Festina cycling team just before the start of the race. A resulting investigation revealed systematic doping involving many teams in the Tour de France. Hotels where teams were staying were raided and searched by police, confessions were made by several retired and current riders, and team personnel were arrested or detained. Several teams withdrew completely from the race.

Erythropoiesis-stimulating agents (ESA) are medications which stimulate the bone marrow to make red blood cells. They are used to treat anemia due to end stage kidney disease, chemotherapy, major surgery, or certain treatments in HIV/AIDS. In these situations they decrease the need for blood transfusions. The different agents are more or less equivalent. They are given by injection.
Epoetin beta (INN), sold under the brand name Neorecormon among others, is a synthetic, recombinant form of erythropoietin, a protein that promotes the production of red blood cells. It is an erythropoiesis-stimulating agent (ESA) that is used to treat anemia, commonly associated with chronic kidney failure and cancer chemotherapy.

The 2008 Giro d'Italia was the 91st running of the Giro d'Italia, one of cycling's Grand Tours. It began in Palermo on 10 May and ended in Milan on 1 June. Twenty-two teams entered the race, which was won by Spaniard Alberto Contador of the Astana cycling team. Second and third respectively were Italians Riccardo Riccò and Marzio Bruseghin.
Carlo Santuccione was an Italian sports doctor who was also known as Ali the Chemist. Santuccione worked with Francesco Conconi at the University of Ferrara in Italy at the Centro Studi Biomedici Applicati allo Sport or Biomedical Research Institute.
Matteo Priamo is an Italian professional road bicycle racer, previously of UCI Professional Continental team CSF Group–Navigare.

The French Anti-Doping Agency is an independent public authority formed in 2006 and charged with ensuring that participants in sports in France do not violate rules regarding doping.
Continuous erythropoietin receptor activator (CERA) is the generic term for drugs in a new class of third-generation erythropoiesis-stimulating agents (ESAs). In the media, these agents are commonly referred to as 'EPO', short for erythropoietin. CERAs have an extended half-life and a mechanism of action that promotes increased stimulation of erythropoietin receptors compared with other ESAs.
Peginesatide, developed by Affymax and Takeda, is an erythropoietic agent, a functional analog of erythropoietin.
CSL Vifor is a global specialty pharmaceuticals company in the treatment areas of iron deficiency, dialysis, nephrology & rare disease. It is headquartered in Switzerland and consists of CSL Vifor, Vifor Fresenius Medical Care Renal Pharma (VFMCRP) and Sanifit Therapeutics.

Marion Sicot is a French racing cyclist, who is suspended from the sport until 2024, following a positive test for erythropoietin (EPO). She finished ninth at the 2014 Grand Prix de Plumelec-Morbihan Dames and seventh at the 2015 Grand Prix de Plumelec-Morbihan Dames.






