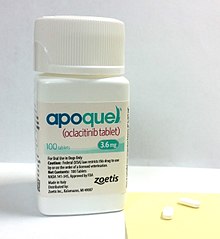
Janus kinase (JAK) is a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK-STAT pathway. They were initially named "just another kinase" 1 and 2, but were ultimately published as "Janus kinase". The name is taken from the two-faced Roman god of beginnings, endings and duality, Janus, because the JAKs possess two near-identical phosphate-transferring domains. One domain exhibits the kinase activity, while the other negatively regulates the kinase activity of the first.

Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved can vary from small to covering the entire body. Dermatitis is often called eczema, and the difference between those terms is not standardized.

Itch is a sensation that causes a strong desire or reflex to scratch. Itches have resisted many attempts to be classified as any one type of sensory experience. Itches have many similarities to pain, and while both are unpleasant sensory experiences, their behavioral response patterns are different. Pain creates a withdrawal reflex, whereas itches leads to a scratch reflex.
Antipruritics, abirritants, or anti-itch drugs, are medications that inhibit the itching often associated with sunburns, allergic reactions, eczema, psoriasis, chickenpox, fungal infections, insect bites and stings like those from mosquitoes, fleas, and mites, and contact dermatitis and urticaria caused by plants such as poison ivy or stinging nettle. It can also be caused by chronic kidney disease and related conditions.
Skin disorders are among the most common health problems in dogs, and have many causes. The condition of a dog's skin and coat is also an important indicator of its general health. Skin disorders of dogs vary from acute, self-limiting problems to chronic or long-lasting problems requiring life-time treatment. Skin disorders may be primary or secondary in nature, making diagnosis complicated.

Atopic dermatitis (AD), also known as atopic eczema, is a long-term type of inflammation of the skin (dermatitis). It results in itchy, red, swollen, and cracked skin. Clear fluid may come from the affected areas, which can thicken over time. AD may also simply be called eczema, a term that generally refers to a larger group of skin conditions.

Avenanthramides are a group of phenolic alkaloids found mainly in oats, but also present in white cabbage butterfly eggs, and in fungus-infected carnation. A number of studies demonstrate that these natural products have anti-inflammatory, antioxidant, anti-itch, anti-irritant, and antiatherogenic activities. Oat kernel extracts with standardized levels of avenanthramides are used for skin, hair, baby, and sun care products. The name avenanthramides was coined by Collins when he reported the presence of these compounds in oat kernels. It was later found that three avenanthramides were the open-ring amides of avenalumins I, II, and III, which were previously reported as oat phytoalexins by Mayama and co-workers.
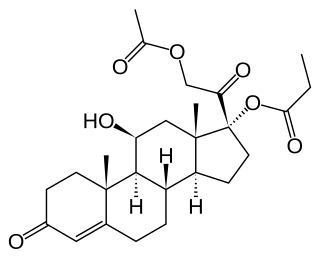
Hydrocortisone aceponate is a veterinary corticosteroid that is used in form of creams for the treatment of various dermatoses. It is an ester of hydrocortisone (cortisol) with acetic acid and propionic acid.

Interleukin-31 (IL-31) is a protein that in humans is encoded by the IL31 gene that resides on chromosome 12. IL-31 is an inflammatory cytokine that helps trigger cell-mediated immunity against pathogens. It has also been identified as a major player in a number of chronic inflammatory diseases, including atopic dermatitis.

Non-receptor tyrosine-protein kinase TYK2 is an enzyme that in humans is encoded by the TYK2 gene.

Tyrosine-protein kinase JAK3 is a tyrosine kinase enzyme that in humans is encoded by the JAK3 gene.

JAK1 is a human tyrosine kinase protein essential for signaling for certain type I and type II cytokines. It interacts with the common gamma chain (γc) of type I cytokine receptors, to elicit signals from the IL-2 receptor family, the IL-4 receptor family, the gp130 receptor family. It is also important for transducing a signal by type I (IFN-α/β) and type II (IFN-γ) interferons, and members of the IL-10 family via type II cytokine receptors. Jak1 plays a critical role in initiating responses to multiple major cytokine receptor families. Loss of Jak1 is lethal in neonatal mice, possibly due to difficulties suckling. Expression of JAK1 in cancer cells enables individual cells to contract, potentially allowing them to escape their tumor and metastasize to other parts of the body.
A Janus kinase inhibitor, also known as JAK inhibitor or jakinib, is a type of immune modulating medication, which inhibits the activity of one or more of the Janus kinase family of enzymes, thereby interfering with the JAK-STAT signaling pathway in lymphocytes.

Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, polyarticular course juvenile idiopathic arthritis, and ulcerative colitis. It is a janus kinase (JAK) inhibitor, discovered and developed by the National Institutes of Health and Pfizer.

Ruxolitinib, sold under the brand name Jakafi among others, is a medication used for the treatment of intermediate or high-risk myelofibrosis, a type of myeloproliferative neoplasm that affects the bone marrow; polycythemia vera, when there has been an inadequate response to or intolerance of hydroxyurea; and steroid-refractory acute graft-versus-host disease. Ruxolitinib is a Janus kinase inhibitor. It was developed and marketed by Incyte Corp in the US under the brand name Jakafi, and by Novartis elsewhere in the world, under the brand name Jakavi.
Adelmidrol is an anti-inflammatory ethanolamide derivative of azelaic acid.

Janus kinase 3 inhibitors, also called JAK3 inhibitors, are a new class of immunomodulatory agents that inhibit Janus kinase 3. They are used for the treatment of autoimmune diseases. The Janus kinases are a family of four nonreceptor tyrosine-protein kinases, JAK1, JAK2, JAK3, and TYK2. They signal via the JAK/STAT pathway, which is important in regulating the immune system. Expression of JAK3 is largely restricted to lymphocytes, while the others are ubiquitously expressed, so selective targeting of JAK3 over the other JAK isozymes is attractive as a possible treatment of autoimmune diseases.
Lokivetmab, trade name Cytopoint, is a monoclonal antibody used to treat atopic dermatitis in dogs. It acts against interleukin 31 (IL-31), which is a cytokine involved in causing itchiness (pruritus). Lokivetmab is administered by subcutaneous injection; each dose is effective for four to eight weeks.

Abrocitinib, sold under the brand name Cibinqo, is a medication used for the treatment of atopic dermatitis (eczema). It is a Janus kinase inhibitor and it was developed by Pfizer. It is taken by mouth.
Brian S. Kim is the Sol and Clara Kest Professor, Vice Chair of Research, and Site Chair of Mount Sinai West and Morningside in the Kimberly and Eric J. Waldman Department of Dermatology at Icahn School of Medicine at Mount Sinai. He is also Director of the Mark Lebwohl Center for Neuroinflammation and Sensation.