
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.

The interleukin 4 is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13.

Interferon gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. Wheelock as a product of human leukocytes stimulated with phytohemagglutinin, and by others as a product of antigen-stimulated lymphocytes. It was also shown to be produced in human lymphocytes. or tuberculin-sensitized mouse peritoneal lymphocytes challenged with Mantoux test (PPD); the resulting supernatants were shown to inhibit growth of vesicular stomatitis virus. Those reports also contained the basic observation underlying the now widely employed IFN-γ release assay used to test for tuberculosis. In humans, the IFN-γ protein is encoded by the IFNG gene.
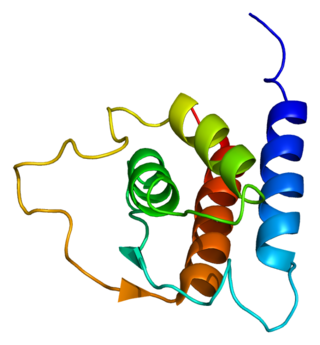
Interleukin 13 (IL-13) is a protein that in humans is encoded by the IL13 gene. IL-13 was first cloned in 1993 and is located on chromosome 5q31.1 with a length of 1.4kb. It has a mass of 13 kDa and folds into 4 alpha helical bundles. The secondary structural features of IL-13 are similar to that of Interleukin 4 (IL-4); however it only has 25% sequence identity to IL-4 and is capable of IL-4 independent signaling. IL-13 is a cytokine secreted by T helper type 2 (Th2) cells, CD4 cells, natural killer T cell, mast cells, basophils, eosinophils and nuocytes. Interleukin-13 is a central regulator in IgE synthesis, goblet cell hyperplasia, mucus hypersecretion, airway hyperresponsiveness, fibrosis and chitinase up-regulation. It is a mediator of allergic inflammation and different diseases including asthma.
Interleukin 33 (IL-33) is a protein that in humans is encoded by the IL33 gene.

Interleukin-25 (IL-25) – also known as interleukin-17E (IL-17E) – is a protein that in humans is encoded by the IL25 gene on chromosome 14. IL-25 was discovered in 2001 and is made up of 177 amino acids.

Interleukin 17 family is a family of pro-inflammatory cystine knot cytokines. They are produced by a group of T helper cell known as T helper 17 cell in response to their stimulation with IL-23. Originally, Th17 was identified in 1993 by Rouvier et al. who isolated IL17A transcript from a rodent T-cell hybridoma. The protein encoded by IL17A is a founding member of IL-17 family. IL17A protein exhibits a high homology with a viral IL-17-like protein encoded in the genome of T-lymphotropic rhadinovirus Herpesvirus saimiri. In rodents, IL-17A is often referred to as CTLA8.

Interleukin 19 (IL-19) is an immunosuppressive protein that belongs to the IL-10 cytokine subfamily.

An alveolar macrophage, pulmonary macrophage, is a type of macrophage, a professional phagocyte, found in the airways and at the level of the alveoli in the lungs, but separated from their walls.

Chemokine ligand 18 (CCL18) is a small cytokine belonging to the CC chemokine family. The functions of CCL18 have been well studied in laboratory settings, however the physiological effects of the molecule in living organisms have been difficult to characterize because there is no similar protein in rodents that can be studied. The receptor for CCL18 has been identified in humans only recently, which will help scientists understand the molecule's role in the body.

Signal transducer and activator of transcription 6 (STAT6) is a transcription factor that belongs to the Signal Transducer and Activator of Transcription (STAT) family of proteins. The proteins of STAT family transmit signals from a receptor complex to the nucleus and activate gene expression. Similarly as other STAT family proteins, STAT6 is also activated by growth factors and cytokines. STAT6 is mainly activated by cytokines interleukin-4 and interleukin-13.

C-C chemokine receptor type 9 is a protein that in humans is encoded by the CCR9 gene. This gene is mapped to the chemokine receptor gene cluster region. Two alternatively spliced transcript variants have been described.
Cytokine receptor-like factor 2 is a protein that in humans is encoded by the CRLF2 gene. It forms a ternary signaling complex with TSLP and interleukin-7 receptor-α, capable of stimulating cell proliferation through activation of STAT3, STAT5 and JAK2 pathways and is implicated in the development of the hematopoietic system. Rearrangement of this gene with immunoglobulin heavy chain gene (IGH), or with P2Y purinoceptor 8 gene (P2RY8) is associated with B-progenitor- and Down syndrome- acute lymphoblastic leukemia (ALL).

Long-term close-knit interactions between symbiotic microbes and their host can alter host immune system responses to other microorganisms, including pathogens, and are required to maintain proper homeostasis. The immune system is a host defense system consisting of anatomical physical barriers as well as physiological and cellular responses, which protect the host against harmful microorganisms while limiting host responses to harmless symbionts. Humans are home to 1013 to 1014 bacteria, roughly equivalent to the number of human cells, and while these bacteria can be pathogenic to their host most of them are mutually beneficial to both the host and bacteria.

Mucosal immunology is the study of immune system responses that occur at mucosal membranes of the intestines, the urogenital tract, and the respiratory system. The mucous membranes are in constant contact with microorganisms, food, and inhaled antigens. In healthy states, the mucosal immune system protects the organism against infectious pathogens and maintains a tolerance towards non-harmful commensal microbes and benign environmental substances. Disruption of this balance between tolerance and deprivation of pathogens can lead to pathological conditions such as food allergies, irritable bowel syndrome, susceptibility to infections, and more.
Interleukin 1 receptor-like 1, also known as IL1RL1 and ST2, is a protein that in humans is encoded by the IL1RL1 gene.
Innate lymphoid cells (ILCs) are the most recently discovered family of innate immune cells, derived from common lymphoid progenitors (CLPs). In response to pathogenic tissue damage, ILCs contribute to immunity via the secretion of signalling molecules, and the regulation of both innate and adaptive immune cells. ILCs are primarily tissue resident cells, found in both lymphoid, and non- lymphoid tissues, and rarely in the blood. They are particularly abundant at mucosal surfaces, playing a key role in mucosal immunity and homeostasis. Characteristics allowing their differentiation from other immune cells include the regular lymphoid morphology, absence of rearranged antigen receptors found on T cells and B cells, and phenotypic markers usually present on myeloid or dendritic cells.

ILC2 cells, or type 2 innate lymphoid cells are a type of innate lymphoid cell. Not to be confused with the ILC. They are derived from common lymphoid progenitor and belong to the lymphoid lineage. These cells lack antigen specific B or T cell receptor because of the lack of recombination activating gene. ILC2s produce type 2 cytokines and are involved in responses to helminths, allergens, some viruses, such as influenza virus and cancer.
In cell biology, TH9 cells are a sub-population of CD4+T cells that produce interleukin-9 (IL-9). They play a role in defense against helminth infections, in allergic responses, in autoimmunity, and tumor suppression.
Type 2 inflammation is a pattern of immune response. Its physiological function is to defend the body against helminths, but a dysregulation of the type 2 inflammatory response has been implicated in the pathophysiology of several diseases.






















