Related Research Articles

Macular degeneration, also known as age-related macular degeneration, is a medical condition which may result in blurred or no vision in the center of the visual field. Early on there are often no symptoms. Over time, however, some people experience a gradual worsening of vision that may affect one or both eyes. While it does not result in complete blindness, loss of central vision can make it hard to recognize faces, drive, read, or perform other activities of daily life. Visual hallucinations may also occur but these do not represent a mental illness.
The Age-Related Eye Disease Study (AREDS) was a clinical trial sponsored by the National Eye Institute, one of the National Institutes of Health in the United States. The study was designed to
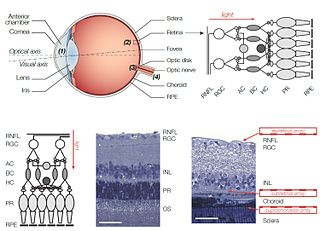
Retinal prostheses for restoration of sight to patients blinded by retinal degeneration are being developed by a number of private companies and research institutions worldwide. The system is meant to partially restore useful vision to people who have lost their photoreceptors due to retinal diseases such as retinitis pigmentosa (RP) or age-related macular degeneration (AMD). Three types of retinal implants are currently in clinical trials: epiretinal, subretinal, and suprachoroidal. Retinal implants introduce visual information into the retina by electrically stimulating the surviving retinal neurons. So far, elicited percepts had rather low resolution, and may be suitable for light perception and recognition of simple objects.
Stargardt disease is the most common inherited single-gene retinal disease. It usually has an autosomal recessive inheritance caused by mutations in the ABCA4 gene. Rarely it has an autosomal dominant inheritance due to defects with ELOVL4 or PROM1 genes. It is characterised by macular degeneration that begins in childhood, adolescence or adulthood, resulting in progressive loss of vision.
Ranibizumab is a monoclonal antibody fragment (Fab) created from the same parent mouse antibody as bevacizumab. It is an anti-angiogenic that has been approved to treat the "wet" type of age-related macular degeneration, a common form of age-related vision loss.

Pegaptanib sodium injection is an anti-angiogenic medicine for the treatment of neovascular (wet) age-related macular degeneration (AMD). It was discovered by NeXstar Pharmaceuticals and licensed in 2000 to EyeTech Pharmaceuticals, now OSI Pharmaceuticals, for late stage development and marketing in the United States. Gilead Sciences continues to receive royalties from the drugs licensing. Outside the US pegaptanib is marketed by Pfizer. Approval was granted by the U.S. Food and Drug Administration (FDA) in December 2004.
Astellas Institute for Regenerative Medicine is a subsidiary of Astellas Pharma located in Marlborough, Massachusetts, US, developing stem cell therapies with a focus on diseases that cause blindness. It was formed in 1994 as a company named Advanced Cell Technology, Incorporated (ACT), which was renamed to Ocata Therapeutics in November 2014. In February 2016 Ocata acquired by Astellas for $379 million. which was finally completed in February 2016.

A maculopathy is any pathological condition of the macula, an area at the centre of the retina that is associated with highly sensitive, accurate vision.

The mission of the Foundation Fighting Blindness is to fund research that will lead to the prevention, treatment and cures for the entire spectrum of retinal degenerative diseases, including retinitis pigmentosa, macular degeneration, Usher syndrome, Stargardt disease and related conditions. These diseases, which affect more than 10 million Americans and millions more throughout the world, often lead to severe vision loss or complete blindness.
Jeffrey W. Berger was a vitreoretinal surgeon and engineer.

Macular telangiectasia describes two distinct retinal diseases affecting the macula of the eye, macular telangiectasia type 1 and macular telangiectasia type 2.
Nesvacumab is an experimental monoclonal antibody originally designed for the treatment of cancer. It targets the protein angiopoietin 2. As of May 2017, it is in Phase II clinical trials for the treatment of diabetic macular edema.
Anti–vascular endothelial growth factor therapy, also known as anti-VEGF therapy or anti-VEGF medication, is the use of medications that block vascular endothelial growth factor. This is done in the treatment of certain cancers and in age-related macular degeneration. They can involve monoclonal antibodies such as bevacizumab, antibody derivatives such as ranibizumab (Lucentis), or orally-available small molecules that inhibit the tyrosine kinases stimulated by VEGF: lapatinib, sunitinib, sorafenib, axitinib, and pazopanib.

Emixustat is a small molecule notable for its establishment of a new class of compounds known as visual cycle modulators (VCMs). Formulated as the hydrochloride salt, emixustat hydrochloride, it is the first synthetic medicinal compound shown to affect retinal disease processes when taken by mouth. Emixustat was invented by the British-American chemist, Ian L. Scott, and is currently in Phase 3 trials for dry, age-related macular degeneration (AMD).
Brolucizumab, sold under the trade name Beovu, is a humanized single-chain antibody fragment for the treatment of neovascular (wet) age-related macular degeneration (AMD).
Geographic atrophy (GA), also known as atrophic age-related macular degeneration (AMD) or advanced dry AMD, is an advanced form of age-related macular degeneration that can result in the progressive and irreversible loss of retina which can lead to a loss of visual function over time. It is estimated that GA affects >5 million people worldwide and approximately 1 million patients in the US, which is similar to the prevalence of neovascular (wet) AMD, the other advanced form of the disease.
Faricimab (INN) is a bispecific monoclonal antibody that is being investigated for diabetic macular edema.

Paul A. Sieving is the current Director of the National Eye Institute, part of the U.S. National Institutes of Health. Prior to joining the NIH in 2001, he served on the faculty of the University of Michigan Medical School as the Paul R. Lichter Professor of Ophthalmic Genetics. He also was the founding director of the Center for Retinal and Macular Degeneration in the university's Department of Ophthalmology and Visual Sciences.
Professor Robyn Guymer was awarded an Elizabeth Blackburn Fellow from the NHMRC, and works in Ophthalmology at Melbourne University. Guymer is a senior retinal specialist within the Royal Victorian Eye and Ear Hospital, and is the Deputy Director, Centre for Eye Research Australia. She works in age-related macular degeneration as a clinician, academic and researcher, and has used nano-lasers to treat Age-related Macular Degeneration.

Indocyanine green angiography (ICGA) is a diagnostic procedure used to examine choroidal blood flow and associated pathology. Indocyanine green (ICG) is a water soluble cyanine dye which shows fluorescence in near-infrared range, with peak spectral absorption of 800-810 nm in blood. The near infrared light used in ICGA penetrates ocular pigments such as melanin and xanthophyll, as well as exudates and thin layers of sub-retinal vessels. Age-related macular degeneration is the third main cause of blindness worldwide, and it is the leading cause of blindness in industrialized countries. Indocyanine green angiography is widely used to study choroidal neovascularization in patients with exudative age-related macular degeneration. In nonexudative AMD, ICGA is used in classification of drusen and associated subretinal deposits.
References
- ↑ World Health Organization (2012). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 107" (PDF). WHO Drug Information. 26 (2).
- ↑ "Statement On A Nonproprietary Name Adopted By The USAN Council - Lampalizumab" (PDF). American Medical Association.
- ↑ "Roche provides update on first lampalizumab phase III study for geographic atrophy, an advanced form of age-related macular degeneration". www.roche.com. Retrieved 2017-09-14.
- ↑ "Statement on Chroma Study". www.roche.com. Retrieved 2017-11-10.
- ↑ Dolgin E (November 2017). "Age-related macular degeneration foils drugmakers". Nature Biotechnology. 35 (11): 1000–1001. doi:10.1038/nbt1117-1000. PMID 29121027. S2CID 9682962.
| This monoclonal antibody–related article is a stub. You can help Wikipedia by expanding it. |