Related Research Articles

Hives, also known as urticaria, is a kind of skin rash with red, raised, itchy bumps. Hives may burn or sting. The patches of rash may appear on different body parts, with variable duration from minutes to days, and does not leave any long-lasting skin change. Fewer than 5% of cases last for more than six weeks. The condition frequently recurs.
Omalizumab, sold under the brand name Xolair, is a medication to treat asthma, nasal polyps, and urticaria (hives).
Lumiliximab is an IgG1k monoclonal antibody that targets CD23. It acts as an immunomodulator and was awarded orphan drug status and fast track designation by the FDA.
Albinterferon is a recombinant fusion protein drug consisting of interferon alpha (IFN-α) linked to human albumin. Conjugation to human albumin prolongs the half-life of the IFN-α to about 6 days, allowing to dose it every two to four weeks.
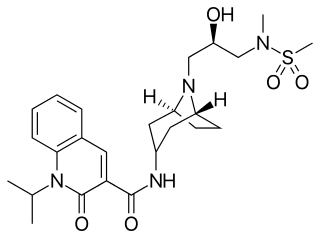
Velusetrag (INN, USAN; previously known as TD-5108) is an experimental drug candidate for the treatment of gastric neuromuscular disorders including gastroparesis, and lower gastrointestinal motility disorders including chronic idiopathic constipation and irritable bowel syndrome. It is a potent, selective, high efficacy 5-HT4 receptor serotonin agonist being developed by Theravance Biopharma and Alfa Wassermann. Velusetrag demonstrates less selectivity for other serotonin receptors, such as 5-HT2 and 5-HT3, to earlier generation 5-HT agonists like cisapride and tegaserod.

Tralokinumab sold under the brand names Adtralza (EU/UK) and Adbry (US) among others, is a human monoclonal antibody used for the treatment of atopic dermatitis. Tralokinumab targets the cytokine interleukin 13.
Quilizumab (INN) is a humanized monoclonal antibody designed for the treatment of asthma. It binds to IGHE.
Tildrakizumab, sold under the brand names Ilumya and Ilumetri, is a monoclonal antibody designed for the treatment of immunologically mediated inflammatory disorders. It is approved for the treatment of adult patients with moderate-to-severe plaque psoriasis in the United States and the European Union.

Chronic spontaneous urticaria(CSU) also known as Chronic idiopathic urticaria(CIU) is defined by the presence of wheals, angioedema, or both for more than six weeks. Chronic spontaneous urticaria can be characterized by angioedema, excruciatingly itchy recurrent hives, or both. Chronic urticaria patients were found to have a higher prevalence of various autoimmune diseases. Many patients with chronic spontaneous urticaria report that certain triggers, such as stress, infections, specific foods, or nonsteroidal anti-inflammatory drug use, aggravate their condition.
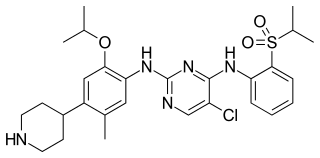
Ceritinib is a prescription-only drug used for the treatment of non-small cell lung cancer (NSCLC). It was developed by Novartis and received FDA approval for use in April 2014..Ceritinib is also sold under the brand name Spexib in few countries by Novartis.

Serlopitant (INN, codenamed VPD-737) is a drug which acts as an NK1 receptor antagonist. It was assessed in clinical trials for the treatment of urinary incontinence and overactive bladder, but while it was superior to placebo it provided no advantage over existing approved drugs, and was not approved for further development for this indication. Serlopitant is now undergoing clinical trials for the treatment of chronic pruritus (itch)

Ipragliflozin is a pharmaceutical drug for treatment of type 2 diabetes. Ipragliflozin, jointly developed by Astellas Pharma and Kotobuki Pharmaceutical, was approved in Japan on January 17, 2014, and in Russia on May 22, 2019.
Brolucizumab sold under trade name Beovu among others, is a humanized single-chain antibody fragment for the treatment of neovascular (wet) age-related macular degeneration (AMD).

Erenumab, sold under the brand name Aimovig, is a medication which targets the calcitonin gene-related peptide receptor (CGRPR) for the prevention of migraine. It is administered by subcutaneous injection.
Tse Wen Chang is an immunology researcher, whose career spans across academia and industry. His early research involving the Immunoglobulin E (IgE) pathway and antibody-based therapeutics lead to the development of omalizumab, a medication that has been approved for the treatment of severe allergic asthma and severe chronic spontaneous urticaria. Chang is a cofounder of Tanox, a biopharmaceutical company specialized in anti-IgE therapies for the treatment of allergic diseases. After Tanox's tripartite partnership with Genentech and Novartis was forged in 1996, Chang returned to his alma mater, the National Tsing Hua University in Taiwan and served as the Dean (1996–1999) of the College of Life Sciences. Chang was appointed by the Taiwanese government as President of the Development Center for Biotechnology (DCB) in 2000, and served as a Science and Technology Advisor of the Executive Yuan from 2002 to 2006. From 2006 to 2016, he was tenured as Distinguished Research Fellow at the Genomics Research Center, Academia Sinica. He founded Immunwork, Inc. in 2014.
Ianalumab is a monoclonal antibody that is being investigated for autoimmune hepatitis, multiple sclerosis, pemphigus vulgaris, rheumatoid arthritis, Sjögren syndrome, and systemic lupus erythematosus.
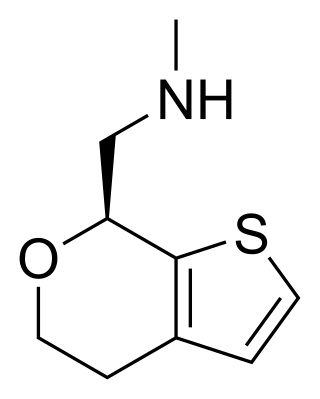
Ulotaront is an investigational antipsychotic that is undergoing clinical trials for the treatment of schizophrenia and Parkinson's disease psychosis. The medication was discovered in collaboration between PsychoGenics Inc. and Sunovion Pharmaceuticals using PsychoGenics' behavior and AI-based phenotypic drug discovery platform, SmartCube. Ulotaront is in Phase III of clinical development.
Lirentelimab is a humanized nonfucosylated monoclonal antibody that targets sialic acid-binding Ig-like lectin 8 (SIGLEC8). In a randomized clinical trial, lirentelimab was found to improve eosinophil counts and symptoms in individuals with eosinophilic gastritis and duodenitis. Adverse reactions include infusion reactions, which are mild to moderate and typically occur following the first infusion.
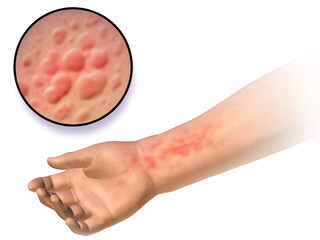
Autoimmune urticaria, also known as chronic autoimmune urticaria, is a type of chronic urticaria characterized by the presence of autoantibodies in the patient's immune system that target the body's own mast cells, leading to episodes of hives (urticaria). This immunologically distinct type of urticaria is considered autoimmune because the immune system, which normally protects the body from foreign organisms, mistakenly attacks the body's own cells, causing inflammation and other symptoms.

Remibrutinib is a small molecule drug that acts as a Bruton's tyrosine kinase (BTK) inhibitor. It is in development for the treatment of chronic spontaneous urticaria. In November 2023, Novartis announced that the compound "demonstrated clinically meaningful and statistically significant reduction in urticaria activity vs placebo" in a Phase III trial.
References
- ↑ Novartis Pharma AG (29 October 2014). "Ligelizumab" (PDF). Statement On A Nonproprietary Name Adopted By The USAN Council. American Medical Association.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 107" (PDF). WHO Drug Information. 26 (2). 2012.
- ↑ Kocatürk E, Maurer M, Metz M, Grattan C (2017-01-10). "Looking forward to new targeted treatments for chronic spontaneous urticaria". Clinical and Translational Allergy. 7: 1. doi: 10.1186/s13601-016-0139-2 . PMC 5223554 . PMID 28078079.
- ↑ Clinical trial number NCT02477332 for "A Multi-center, Randomized, Double-blind, Placebo, and Active-controlled Phase 2b Dose-finding Study of QGE031 as add-on Therapy to Investigate the Efficacy and Safety in Patients With Chronic Spontaneous Urticaria (CSU)" at ClinicalTrials.gov
- ↑ Maurer M, Giménez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. (October 2019). "Ligelizumab for Chronic Spontaneous Urticaria". The New England Journal of Medicine. 381 (14): 1321–1332. doi: 10.1056/NEJMoa1900408 . hdl: 10230/43837 . PMID 31577874.