
Tumor necrosis factor is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain.

Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease. It is given by slow injection into a vein, typically at six- to eight-week intervals.
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses.
A TNF inhibitor is a pharmaceutical drug that suppresses the physiologic response to tumor necrosis factor (TNF), which is part of the inflammatory response. TNF is involved in autoimmune and immune-mediated disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis, hidradenitis suppurativa and refractory asthma, so TNF inhibitors may be used in their treatment. The important side effects of TNF inhibitors include lymphomas, infections, congestive heart failure, demyelinating disease, a lupus-like syndrome, induction of auto-antibodies, injection site reactions, and systemic side effects.

Certolizumab pegol, sold under the brand name Cimzia, is a biopharmaceutical medication for the treatment of Crohn's disease, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is a fragment of a monoclonal antibody specific to tumor necrosis factor alpha (TNF-α) and is manufactured by UCB.

Interleukin 20 (IL20) is a protein that is in humans encoded by the IL20 gene which is located in close proximity to the IL-10 gene on the 1q32 chromosome. IL-20 is a part of an IL-20 subfamily which is a part of a larger IL-10 family.
Zanolimumab is a human monoclonal antibody and an immunosuppressive drug. It was developed with the goal of treatment of rheumatoid arthritis, psoriasis, melanoma, cutaneous and peripheral T-cell lymphoma. Development of the drug was ultimately discontinued with termination of all trials.
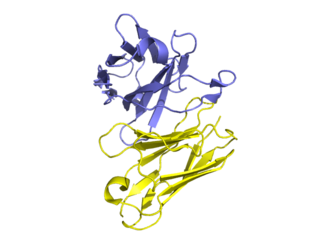
Golimumab is a human monoclonal antibody which is used as an immunosuppressive medication and sold under the brand name Simponi. Golimumab targets tumor necrosis factor alpha (TNF-alpha), a pro-inflammatory molecule and hence is a TNF inhibitor. Profound reduction in C-reactive protein (CRP) levels, interleukin (IL)-6, intercellaular adhesion molecules (ICAM)-1, matrix metalloproteinase (MMP)-3, and vascular endothelial growth factor (VEGF) demonstrates golimumab as an effective modulator of inflammatory markers and bone metabolism.

Lymphotoxin-alpha (LT-α) formerly known as tumor necrosis factor-beta (TNF-β) is a protein that in humans is encoded by the LTA gene. Belonging to the hematopoietic cell line, LT-α exhibits anti-proliferative activity and causes the cellular destruction of tumor cell lines. As a cytotoxic protein, LT-α performs a variety of important roles in immune regulation depending on the form that it is secreted as. Unlike other members of the TNF superfamily, LT-α is only found as a soluble homotrimer, when found at the cell surface it is found only as a heterotrimer with LTβ.

Interferon alpha-1 is a protein that in humans is encoded by the IFNA1 gene.
Clenoliximab (INN) is a monoclonal antibody against CD4. It acts as an immunomodulator and has been investigated for the treatment of rheumatoid arthritis. The drug is a chimeric antibody from Macaca irus and Homo sapiens.
Mavrilimumab is a human monoclonal antibody that inhibits human granulocyte macrophage colony-stimulating factor receptor (GM-CSF-R).
Sirukumab is a human monoclonal antibody designed for the treatment of rheumatoid arthritis. It acts against the proinflammatory cytokine Interleukin 6 (IL-6).
Namilumab is a human monoclonal antibody that targets granulocyte macrophage-colony stimulating factor (GM-CSF)/colony stimulating factor 2 (CSF2) and is currently being researched for application in rheumatoid arthritis (RA) and psoriatic arthritis. Clinical trials investigating the therapeutic utility of Namilumab have include phase I and phase II clinical trials to establish the safety, tolerability and preliminary therapeutic utility of the antibody in plaque psoriasis and rheumatoid arthritis.
Inclacumab (LC1004-002) (INN) is a human monoclonal antibody designed for the treatment of cardiovascular disease.
Quilizumab (INN) is a humanized monoclonal antibody designed for the treatment of asthma. It binds to IGHE.
Fletikumab (NNC0109-0012) (INN) is a monoclonal antibody designed for the treatment of rheumatoid arthritis that targets IL-20.
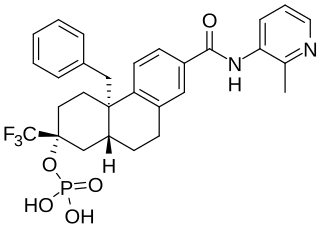
Fosdagrocorat is a nonsteroidal but steroid-like selective glucocorticoid receptor modulator (SGRM) which was under development for the treatment of rheumatoid arthritis but was never marketed. It is the C2 dihydrogen phosphate ester of dagrocorat, and acts as a prodrug of dagrocorat with improved pharmacokinetics. The drug reached phase II clinical trials prior to the discontinuation of its development.
Otilimab is a fully human antibody which has been developed by the biotechnology company MorphoSys. It can also be referred to as HuCAL antibody, HuCAL standing for Human Combinatorial Antibody Library and being a technology used to generate monoclonal antibodies. Otilimab is directed against the granulocyte-macrophage colony stimulating factor (GM-CSF), a monomeric glycoprotein functioning as a cytokine promoting both proliferation and activation of macrophages and neutrophils.
Ianalumab is a monoclonal antibody that is being investigated for autoimmune hepatitis, multiple sclerosis, pemphigus vulgaris, rheumatoid arthritis, Sjögren syndrome, and systemic lupus erythematosus.







