Related Research Articles

The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.

Cis–trans isomerism, also known as geometric isomerism, describes certain arrangements of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.

Paraffin wax is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and begins to melt above approximately 37 °C (99 °F), and its boiling point is above 370 °C (698 °F). Common applications for paraffin wax include lubrication, electrical insulation, and candles; dyed paraffin wax can be made into crayons. It is distinct from kerosene and other petroleum products that are sometimes called paraffin.

In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.

A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.
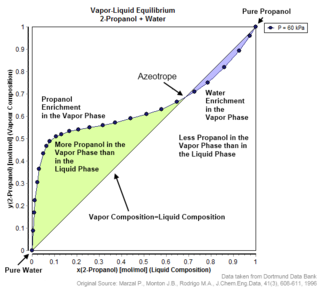
An azeotrope or a constant heating point mixture is a mixture of two or more components in fluidic states whose proportions cannot be altered or changed by simple distillation. This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Azeotropic mixture behavior is important for fluid separation processes.
In chemistry, colligative properties are those properties of solutions that depend on the ratio of the number of solute particles to the number of solvent particles in a solution, and not on the nature of the chemical species present. The number ratio can be related to the various units for concentration of a solution such as molarity, molality, normality (chemistry), etc. The assumption that solution properties are independent of nature of solute particles is exact only for ideal solutions, which are solutions that exhibit thermodynamic properties analogous to those of an ideal gas, and is approximate for dilute real solutions. In other words, colligative properties are a set of solution properties that can be reasonably approximated by the assumption that the solution is ideal.

Freezing-point depression is a drop in the maximum temperature at which a substance freezes, caused when a smaller amount of another, non-volatile substance is added. Examples include adding salt into water, alcohol in water, ethylene or propylene glycol in water, adding copper to molten silver, or the mixing of two solids such as impurities into a finely powdered drug.

Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer, in which case pentanes refers to a mixture of them; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.

A rotary evaporator (rotavap) is a device used in chemical laboratories for the efficient and gentle removal of solvents from samples by evaporation. When referenced in the chemistry research literature, description of the use of this technique and equipment may include the phrase "rotary evaporator", though use is often rather signaled by other language.
Petroleum ether is the petroleum fraction consisting of aliphatic hydrocarbons and boiling in the range 35–60 °C, and commonly used as a laboratory solvent. Despite the name, petroleum ether is not an ether; the term is used only figuratively, signifying extreme lightness and volatility.
This is a list of the various reported boiling points for the elements, with recommended values to be used elsewhere on Wikipedia.

In chemistry, hydrogen halides are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, astatine, or tennessine. All known hydrogen halides are gases at Standard Temperature and Pressure.

In thermodynamics, a critical point is the end point of a phase equilibrium curve. One example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions under which a liquid and its vapor can coexist. At higher temperatures, the gas cannot be liquefied by pressure alone. At the critical point, defined by a critical temperatureTc and a critical pressurepc, phase boundaries vanish. Other examples include the liquid–liquid critical points in mixtures, and the ferromagnet–paramagnet transition in the absence of an external magnetic field.
Boiling-point elevation is the phenomenon whereby the boiling point of a liquid will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. The boiling point can be measured accurately using an ebullioscope.
François-Marie Raoult was a French chemist who conducted research into the behavior of solutions, especially their physical properties.
Higher alkanes are alkanes having nine or more carbon atoms. Nonane is the lightest alkane to have a flash point above 25 °C, and is not classified as dangerously flammable.
In thermodynamics, the ebullioscopic constantKb relates molality b to boiling point elevation. It is the ratio of the latter to the former:
This page contains tables of azeotrope data for various binary and ternary mixtures of solvents. The data include the composition of a mixture by weight, the boiling point (b.p.) of a component, the boiling point of a mixture, and the specific gravity of the mixture. Boiling points are reported at a pressure of 760 mm Hg unless otherwise stated. Where the mixture separates into layers, values are shown for upper (U) and lower (L) layers.
References
- 1 2 3 4 5 6 7 8 9 Eastman. E.D. and Rollefson, G.K. Physical Chemistry 1947 ed. McGraw-Hill p307
- 1 2 3 4 5 6 Pauling, Linus: General Chemistry 1970 ed. Dover Publications pp459-460
- ↑ Moore, Walter J. Physical Chemistry 1962 ed. Prentice Hall p132
- ↑ "Boiling Point of Gases, Liquids & Solids | Toolbox | AMERICAN ELEMENTS ®".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "P-CHLOROBENZOTRIFLUORIDE".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".
- ↑ "Solvent Boiling Points Chart -".