Aortic stenosis is the narrowing of the exit of the left ventricle of the heart, such that problems result. It may occur at the aortic valve as well as above and below this level. It typically gets worse over time. Symptoms often come on gradually with a decreased ability to exercise often occurring first. If heart failure, loss of consciousness, or heart related chest pain occur due to AS the outcomes are worse. Loss of consciousness typically occurs with standing or exercising. Signs of heart failure include shortness of breath especially when lying down, at night, or with exercise, and swelling of the legs. Thickening of the valve without narrowing is known as aortic sclerosis.

Mitral valve prolapse (MVP) is a valvular heart disease characterized by the displacement of an abnormally thickened mitral valve leaflet into the left atrium during systole. It is the primary form of myxomatous degeneration of the valve. There are various types of MVP, broadly classified as classic and nonclassic. In severe cases of classic MVP, complications include mitral regurgitation, infective endocarditis, congestive heart failure, and, in rare circumstances, cardiac arrest.

Mitral stenosis is a valvular heart disease characterized by the narrowing of the opening of the mitral valve of the heart. It is almost always caused by rheumatic valvular heart disease. Normally, the mitral valve is about 5 cm2 during diastole. Any decrease in area below 2 cm2 causes mitral stenosis. Early diagnosis of mitral stenosis in pregnancy is very important as the heart cannot tolerate increased cardiac output demand as in the case of exercise and pregnancy. Atrial fibrillation is a common complication of resulting left atrial enlargement, which can lead to systemic thromboembolic complications like stroke.

Aortic regurgitation (AR), also known as aortic insufficiency (AI), is the leaking of the aortic valve of the heart that causes blood to flow in the reverse direction during ventricular diastole, from the aorta into the left ventricle. As a consequence, the cardiac muscle is forced to work harder than normal.
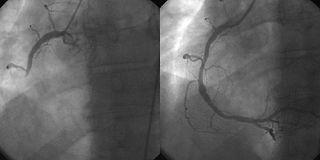
Interventional cardiology is a branch of cardiology that deals specifically with the catheter based treatment of structural heart diseases. Andreas Gruentzig is considered the father of interventional cardiology after the development of angioplasty by interventional radiologist Charles Dotter.

Mitral regurgitation(MR), also known as mitral insufficiency, or mitral incompetence is a form of valvular heart disease in which the mitral valve is insufficient and does not close properly when the heart pumps out blood. It is the abnormal leaking of blood backwards – regurgitation from the left ventricle, through the mitral valve, into the left atrium, when the left ventricle contracts. Mitral regurgitation is the most common form of valvular heart disease.
Aortic valve replacement is a procedure whereby the failing aortic valve of a patient's heart is replaced with an artificial heart valve. The aortic valve may need to be replaced because:

An artificial heart valve is a one-way valve implanted into a person's heart to replace a heart valve that is not functioning properly. Artificial heart valves can be separated into three broad classes: mechanical heart valves, bioprosthetic tissue valves and engineered tissue valves.
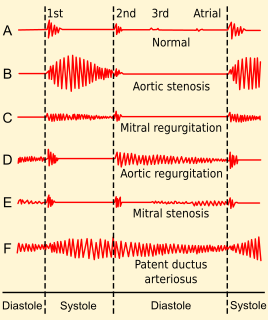
Valvular heart disease is any cardiovascular disease process involving one or more of the four valves of the heart. These conditions occur largely as a consequence of aging, but may also be the result of congenital (inborn) abnormalities or specific disease or physiologic processes including rheumatic heart disease and pregnancy.

Mitral valve repair is a cardiac surgery procedure performed by cardiac surgeons to treat stenosis (narrowing) or regurgitation (leakage) of the mitral valve. The mitral valve is the "inflow valve" for the left side of the heart. Blood flows from the lungs, where it picks up oxygen, through the pulmonary veins, to the left atrium of the heart. After the left atrium fills with blood, the mitral valve allows blood to flow from the left atrium into the heart's main pumping chamber called the left ventricle. It then closes to keep blood from leaking back into the left atrium or lungs when the ventricle contracts (squeezes) to push blood out to the body. It has two flaps, or leaflets, known as cusps.
Percutaneous aortic valve replacement (PAVR), also known as percutaneous aortic valve implantation (PAVI), transcatheter aortic valve implantation (TAVI) or transcatheter aortic valve replacement (TAVR), is the replacement of the aortic valve of the heart through the blood vessels. The replacement valve is delivered via one of several access methods: transfemoral, transapical, subclavian, direct aortic, and transcaval, among others.
Mitral valve replacement is a procedure whereby the diseased mitral valve of a patient's heart is replaced by either a mechanical or tissue (bioprosthetic) valve.
Tricuspid regurgitation (TR), also called tricuspid insufficiency, is a type of valvular heart disease in which the tricuspid valve of the heart, located between the right atrium and right ventricle, does not close completely when the right ventricle contracts (systole). TR allows the blood to flow backwards from the right ventricle to the right atrium, which increases the volume and pressure of the blood both in the right atrium and the right ventricle, which may increase central venous volume and pressure if the backward flow is sufficiently severe.

Lutembacher's syndrome is a very rare form of congenital heart disease that affects one of the chambers of the heart as well as a valve. It is commonly known as both congenital atrial septal defect (ASD) and acquired mitral stenosis (MS). Congenital atrial septal defect refers to a hole being in the septum or wall that separates the two atria; this condition is usually seen in fetuses and infants. Mitral stenosis refers to mitral valve leaflets sticking to each other making the opening for blood to pass from the atrium to the ventricles very small. With the valve being so small, blood has difficulty passing from the left atrium into the left ventricle. Septal defects that may occur with Lutembacher's syndrome include: Ostium primum atrial septal defect or ostium secundum which is more prevalent.
David H. Adams is an American cardiac surgeon and the Marie-Josée and Henry R. Kravis Professor and Chairman of the Department of Cardiothoracic Surgery, Icahn School of Medicine at Mount Sinai Hospital in New York City. Dr. Adams is a recognized leader in the field of heart valve surgery and mitral valve reconstruction. As director of Mount Sinai Mitral Valve Repair Center, he has set national benchmarks with >99% degenerative mitral valve repair rates, while running one of the largest valve repair programs in the United States. Dr. Adams is the co-inventor of 2 mitral valve annuloplasty repair rings – the Carpentier-McCarthy-Adams IMR ETlogix Ring and the Carpentier-Edwards Physio II Annuloplasty Ring, and is a senior consultant with royalty agreements with Edwards Lifesciences. He is also the inventor of the Tri-Ad Adams Tricuspid Annuloplasty ring with a royalty agreement with Medtronic. He is a co-author with Professor Alain Carpentier of the benchmark textbook in mitral valve surgery Carpentier's Reconstructive Valve Surgery. He is also the National Co-Principal Investigator of the FDA pivotal trial of the Medtronic-CoreValve transcatheter aortic valve replacement device.

Left atrial appendage occlusion (LAAO), also referred to as Left atrial appendage closure (LAAC) is a treatment strategy to reduce the risk of left atrial appendage blood clots from entering the bloodstream and causing a stroke in patients with non-valvular atrial fibrillation (AF).
Heart valve repair is a cardiac surgery procedure, carried out to repair one or more faulty heart valves. In some valvular heart diseases repair where possible is preferable to valve replacement. A mechanical heart valve is a replacement valve that is not itself subject to repair.

A hybrid cardiac surgical procedure in a narrow sense is defined as a procedure that combines a conventional, more invasive surgical part with an interventional part, using some sort of catheter-based procedure guided by fluoroscopy imaging in a hybrid operating room (OR) without interruption. The hybrid technique has a reduced risk of surgical complications and has shown decreased recovery time. It can be used to treat numerous heart diseases and conditions and with the increasing complexity of each case, the hybrid surgical technique is becoming more common.

Edwards Lifesciences is an American medical technology company headquartered in Irvine, California, specializing in artificial heart valves and hemodynamic monitoring. It developed the SAPIEN transcatheter aortic heart valve made of cow tissue within a balloon-expandable, cobalt-chromium frame, deployed via catheter. The company has manufacturing facilities at the Irvine headquarters, as well as in Draper, Utah; Costa Rica; the Dominican Republic; Puerto Rico; and Singapore; and is building a new facility due to be completed in 2021 in Limerick, Ireland.
Percutaneous pulmonary valve implantation (PPVI), also known as transcatheter pulmonary valve replacement (TPVR), is the replacement of the pulmonary valve via catheterization through a vein. It is a significantly less invasive procedure in comparison to open heart surgery and is commonly used to treat conditions such as pulmonary atresia.