Glutathione is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine.
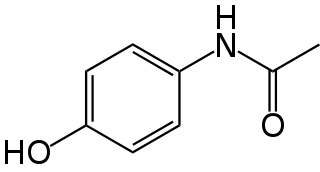
Paracetamol is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely used over the counter medication. Common brand names include Tylenol and Panadol.
Detoxification or detoxication is the physiological or medicinal removal of toxic substances from a living organism, including the human body, which is mainly carried out by the liver. Additionally, it can refer to the period of drug withdrawal during which an organism returns to homeostasis after long-term use of an addictive substance. In medicine, detoxification can be achieved by decontamination of poison ingestion and the use of antidotes as well as techniques such as dialysis and chelation therapy.
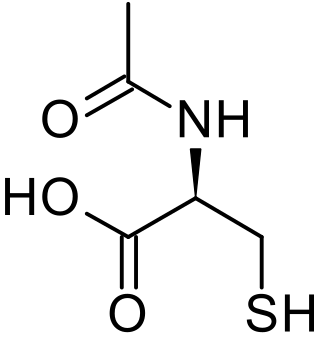
Acetylcysteine, also known as N-acetylcysteine (NAC), is a medication that is used to treat paracetamol overdose and to loosen thick mucus in individuals with chronic bronchopulmonary disorders like pneumonia and bronchitis. It has been used to treat lactobezoar in infants. It can be taken intravenously, by mouth, or inhaled as a mist. Some people use it as a dietary supplement.

Hepatotoxicity implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval.
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds. The study of drug metabolism is called pharmacokinetics.
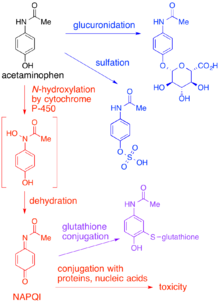
Toxication, toxification or toxicity exaltation is the conversion of a chemical compound into a more toxic form in living organisms or in substrates such as soil or water. The conversion can be caused by enzymatic metabolism in the organisms, as well as by abiotic chemical reactions. While the parent drug are usually less active, both the parent drug and its metabolite can be chemically active and cause toxicity, leading to mutagenesis, teratogenesis, and carcinogenesis. Different classes of enzymes, such as P450-monooxygenases, epoxide hydrolase, or acetyltransferases can catalyze the process in the cell, mostly in the liver.

Acute liver failure is the appearance of severe complications rapidly after the first signs of liver disease, and indicates that the liver has sustained severe damage. The complications are hepatic encephalopathy and impaired protein synthesis. The 1993 classification defines hyperacute as within 1 week, acute as 8–28 days, and subacute as 4–12 weeks; both the speed with which the disease develops and the underlying cause strongly affect outcomes.

Glutathione S-transferases (GSTs), previously known as ligandins, are a family of eukaryotic and prokaryotic phase II metabolic isozymes best known for their ability to catalyze the conjugation of the reduced form of glutathione (GSH) to xenobiotic substrates for the purpose of detoxification. The GST family consists of three superfamilies: the cytosolic, mitochondrial, and microsomal—also known as MAPEG—proteins. Members of the GST superfamily are extremely diverse in amino acid sequence, and a large fraction of the sequences deposited in public databases are of unknown function. The Enzyme Function Initiative (EFI) is using GSTs as a model superfamily to identify new GST functions.

Iproniazid is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class. It is a xenobiotic that was originally designed to treat tuberculosis, but was later most prominently used as an antidepressant drug. However, it was withdrawn from the market because of its hepatotoxicity. The medical use of iproniazid was discontinued in most of the world in the 1960s, but remained in use in France until its discontinuation in 2015.

Sulfur assimilation is the process by which living organisms incorporate sulfur into their biological molecules. In plants, sulfate is absorbed by the roots and then be transported to the chloroplasts by the transipration stream where the sulfur are reduced to sulfide with the help of a series of enzymatic reactions. Furthermore, the reduced sulfur is incorporated into cysteine, an amino acid that is a precursor to many other sulfur-containing compounds. In animals, sulfur assimilation occurs primarily through the diet, as animals cannot produce sulfur-containing compounds directly. Sulfur is incorporated into amino acids such as cysteine and methionine, which are used to build proteins and other important molecules. Besides, With the rapid development of economy, the increase emission of sulfur results in environmental issues, such as acid rain and hydrogen sulfilde.
The King's College Criteria or the King's College Hospital criteria were devised in 1989 to determine if there were any early indices of poor prognosis in patients with acute liver failure. Acute liver failure is defined as the onset of encephalopathy or coagulopathy within 26 weeks of a patient diagnosed with liver disease. Patients with hepatitis B acquired at birth, Wilson's disease and autoimmune hepatitis are included if their disease was identified within the past 26 weeks. These patients are very ill, and have a very high risk of dying of their illness without adequate treatment which may include liver transplantation. It is important that physicians find ways of identifying patients with acute liver failure early in their course who will do poorly, and may require liver transplantation. The King's College Criteria have consistently shown excellent operating characteristics for determining prognosis in these patients. As liver transplantation becomes a more accessible option for patients with acute liver failure, the King's College Criteria serve a role in determining which patients may require transplantation.

Hydrocodone/paracetamol is the combination of the pain medications hydrocodone and paracetamol (acetaminophen). It is used to treat moderate to severe pain. It is taken by mouth. Recreational use is common in the United States.
Glutamate–cysteine ligase (GCL) EC 6.3.2.2), previously known as γ-glutamylcysteine synthetase (GCS), is the first enzyme of the cellular glutathione (GSH) biosynthetic pathway that catalyzes the chemical reaction:
Pharmacotoxicology entails the study of the consequences of toxic exposure to pharmaceutical drugs and agents in the health care field. The field of pharmacotoxicology also involves the treatment and prevention of pharmaceutically induced side effects. Pharmacotoxicology can be separated into two different categories: pharmacodynamics, and pharmacokinetics.

Paracetamol poisoning, also known as acetaminophen poisoning, is caused by excessive use of the medication paracetamol (acetaminophen). Most people have few or non-specific symptoms in the first 24 hours following overdose. These symptoms include feeling tired, abdominal pain, or nausea. This is typically followed by absence of symptoms for a couple of days, after which yellowish skin, blood clotting problems, and confusion occurs as a result of liver failure. Additional complications may include kidney failure, pancreatitis, low blood sugar, and lactic acidosis. If death does not occur, people tend to recover fully over a couple of weeks. Without treatment, death from toxicity occurs 4 to 18 days later.
Methacrylonitrile, MeAN in short, is a chemical compound that is an unsaturated aliphatic nitrile, widely used in the preparation of homopolymers, copolymers, elastomers, and plastics and as a chemical intermediate in the preparation of acids, amides, amines, esters, and other nitriles. MeAN is also used as a replacement for acrylonitrile in the manufacture of an acrylonitrile/butadiene/styrene-like polymer. It is a clear and colorless liquid, that has a bitter almond smell.
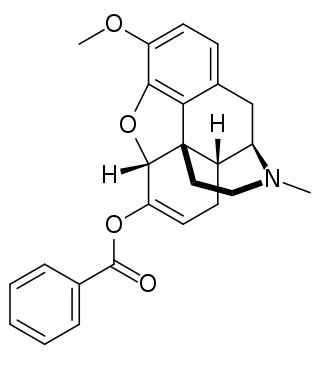
Benzhydrocodone (INN) is an opioid prodrug of the morphinan class. Its chemical structure consists of hydrocodone coupled with benzoic acid. Benzhydrocodone itself is inactive and acts as a prodrug to hydrocodone upon cleavage of the benzoate portion of the molecule.

Glycidamide is an organic compound with the formula H2NC(O)C2H3O. It is a colorless oil. Structurally, it contains adjacent amides and epoxide functional groups. It is a bioactive, potentially toxic or even carcinogenic metabolite of acrylonitrile and acrylamide. It is a chiral molecule.

4-Ipomeanol (4-IPO) is a pulmonary pre-toxin isolated from sweet potatoes infected with the fungus Fusarium solani. One of the 4-IPO metabolites is toxic to the lungs, liver and kidney in humans and animals. This metabolite can covalently bind to proteins, thereby interfering with normal cell processes.