
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.

Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russia rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.

Lead(II) nitrate is an inorganic compound with the chemical formula Pb(NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
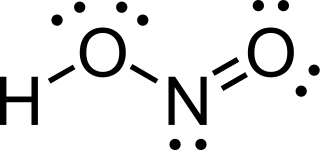
Nitrous acid is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite salts. It was discovered by Carl Wilhelm Scheele, who called it "phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Nitrogen trioxide or nitrate radical is an oxide of nitrogen with formula NO
3, consisting of three oxygen atoms covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and observed as a short-lived component of gas, liquid, or solid systems.
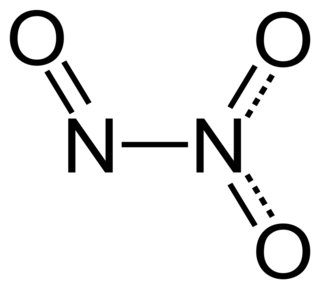
Dinitrogen trioxide is the inorganic compound with the formula N2O3. It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):
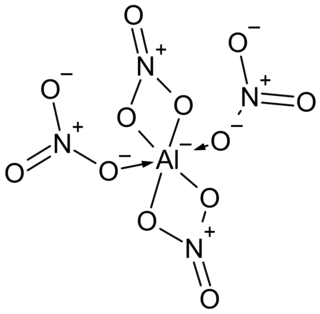
Tetranitratoaluminate is an anion of aluminium and nitrate groups with formula [Al(NO3)4]− that can form salts called tetranitratoaluminates. It is unusual in being a nitrate complex of a light element.

Zirconium nitrate is a volatile anhydrous transition metal nitrate salt of zirconium with formula Zr(NO3)4. It has alternate names of zirconium tetranitrate, or zirconium(IV) nitrate.
Barbara J. Finlayson-Pitts is a Canadian-American atmospheric chemist. She is a professor in the chemistry department at the University of California, Irvine and is the Director of AirUCI Institute. Finlayson-Pitts and James N. Pitts, Jr. are the authors of Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications (1999). She has been a member of the National Academy of Sciences since 2006 and is the laureate for the 2017 Garvan–Olin Medal. In 2016 she co-chaired the National Academy of Science report "The Future of Atmospheric Chemistry Research"
Indium(III) nitrate is a nitrate salt of indium which forms various hydrates. Only the pentahydrate has been crystallographically verified. Other hydrates are also reported in literature, such as the trihydrate.

Nitratoauric acid, hydrogen tetranitratoaurate, or simply called gold(III) nitrate is a crystalline gold compound that forms the trihydrate, HAu(NO3)4·3H2O or more correctly H5O2Au(NO3)4·H2O. This compound is an intermediate in the process of extracting gold. In older literature it is also known as aurinitric acid.

Tin(IV) nitrate is a salt of tin with nitric acid. It is a volatile white solid, subliming at 40 °C under a vacuum. Unlike other nitrates, it reacts with water to produce nitrogen dioxide.

Nitryl chloride is a volatile inorganic compound with formula ClNO2. At standard conditions it is a gas.

A transition metal nitrate complex is a coordination compound containing one or more nitrate ligands. Such complexes are common starting reagents for the preparation of other compounds.
















