Related Research Articles

In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R−O−R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
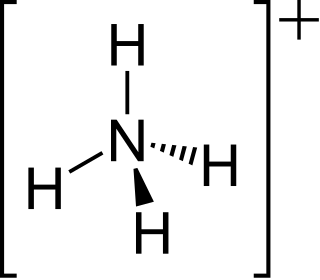
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups.

In organic chemistry, an acetal is a functional group with the connectivity R2C(OR')2. Here, the R groups can be organic fragments or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other or not. Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.
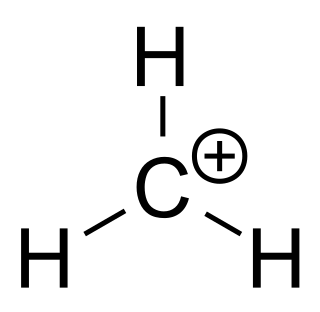
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5 and vinyl C
2H+
3 cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
In chemistry, an oxonium ion is any cation containing an oxygen atom that has three bonds and 1+ formal charge. The simplest oxonium ion is the hydronium ion.

In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.
![<span class="mw-page-title-main">Sulfonium</span> Cation of the form [SR3]+](https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png/320px-%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png)
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula [SR3]+. Together with a negatively-charged counterion, they give sulfonium salts. They are typically colorless solids that are soluble in organic solvent.

A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. But IUPAC confuses coordination number with valence, incorrectly considering carbon in carbenium as trivalent.
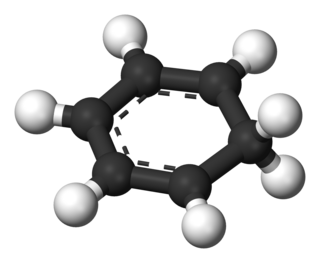
An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution. For historic reasons this complex is also called a Wheland intermediate, after American chemist George Willard Wheland (1907–1976). They are also called sigma complexes. The smallest arenium ion is the benzenium ion, which is protonated benzene.

Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions. This mixture is a superacid that, in terms of corrosiveness, is trillions of times stronger than 100% sulfuric acid in terms of its protonating ability measured by Hammett function. It even protonates some hydrocarbons to afford pentacoordinate carbocations. Like its precursor hydrogen fluoride, it attacks glass, but can be stored in containers lined with PTFE (Teflon) or PFA.
In chemistry, a carbonium ion is any cation that has a pentacoordinated carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium, where the carbon atom is covalently bonded to five hydrogen atoms.
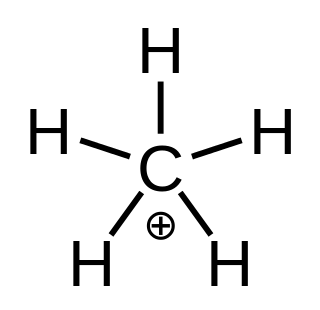
In chemistry, methanium is a complex positive ion with formula [CH5]+ or [CH3(H2)]+, bearing a +1 electric charge. It is a superacid and one of the onium ions, indeed the simplest carbonium ion.

In chemistry, a quaternary compound is a compound consisting of exactly four chemical elements.
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms.
Hydrogen-bridged cations are a type of charged species in which a hydrogen atom is simultaneously bonded to two atoms through partial sigma bonds. While best observable in the presence of superacids at room temperature, spectroscopic evidence has suggested that hydrogen-bridged cations exist in ordinary solvents. These ions have been the subject of debate as they constitute a type of charged species of uncertain electronic structure.

Choline bitartrate is an organic compound with the chemical formula [(CH3)3NCH2CH2OH]+HOOC−CH(OH)−CH(OH)−COO−. It is a white crystalline powder with an acid taste. It is hygroscopic when exposed to air. Modern texts refer to the choline salt of the natural form of tartaric acid, that is, the salt called choline dextrobitartrate, choline (2R,3R)-bitartrate or choline L-(+)-bitartrate.
References
- 1 2 Onium compounds, IUPAC Gold Book
- 1 2 George A. Olah (1998). Onium Ions. John Wiley & Sons. p. 509. ISBN 9780471148777.
- ↑ Advanced Organic Chemistry: Reactions and mechanisms, Maya Shankar Singh, 2007, Dorling Kindersley, ISBN 978-81-317-1107-1
- ↑ Carbonium ion, IUPAC Gold Book
- ↑ RC-82. Cations, Queen Mary University of London)