Related Research Articles
Noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon. From the standpoint of chemistry, the noble gases may be divided into two groups: the relatively reactive krypton, xenon (12.1 eV), and radon (10.7 eV) on one side, and the very unreactive argon (15.8 eV), neon (21.6 eV), and helium (24.6 eV) on the other. Consistent with this classification, Kr, Xe, and Rn form compounds that can be isolated in bulk at or near standard temperature and pressure, whereas He, Ne, Ar have been observed to form true chemical bonds using spectroscopic techniques, but only when frozen into a noble gas matrix at temperatures of 40 K or lower, in supersonic jets of noble gas, or under extremely high pressures with metals.

Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum. It was first synthesized in 1963.

Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:

Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.
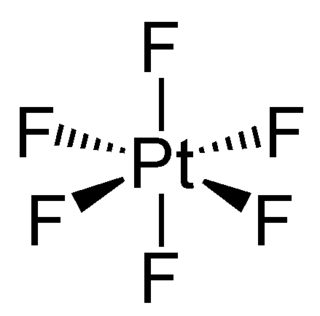
Platinum hexafluoride is the chemical compound with the formula PtF6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong fluorinating agent and one of the strongest oxidants, capable of oxidising xenon and O2. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers.

Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound. Silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Xenon oxytetrafluoride is an inorganic chemical compound. It is a colorless stable liquid with a melting point of −46.2 °C that can be synthesized by partial hydrolysis of XeF
6, or the reaction of XeF
6 with silica or NaNO
3:

Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid. The structure of the KrF2 molecule is linear, with Kr−F distances of 188.9 pm. It reacts with strong Lewis acids to form salts of the KrF+ and Kr
2F+
3 cations.

The dioxygenyl ion, O+
2, is a rarely-encountered oxycation in which both oxygen atoms have a formal oxidation state of +1/2. It is formally derived from oxygen by the removal of an electron:

Manganese tetrafluoride, MnF4, is the highest fluoride of manganese. It is a powerful oxidizing agent and is used as a means of purifying elemental fluorine.

Diethylaminosulfur trifluoride (DAST) is the organosulfur compound with the formula Et2NSF3. This liquid is a fluorinating reagent used for the synthesis of organofluorine compounds. The compound is colourless; older samples assume an orange colour.
Dioxygenyl hexafluoroplatinate is a compound with formula O2PtF6. It is a hexafluoroplatinate of the unusual dioxygenyl cation, O2+, and is the first known compound containing this cation. It can be produced by the reaction of dioxygen with platinum hexafluoride. The fact that PtF
6 is strong enough to oxidise O
2, whose first ionization potential is 12.2 eV, led Neil Bartlett to correctly surmise that it might be able to oxidise xenon (first ionization potential 12.13 eV). This led to the discovery of xenon hexafluoroplatinate, which proved that the noble gases, previously thought to be inert, are able to form chemical compounds.

The tetrafluoroammonium cation is a positively charged polyatomic ion with chemical formula NF+
4. It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane CF
4, trifluoramine oxide ONF
3 and the tetrafluoroborate BF−
4 anion.
A tetrafluoride is a chemical compound with four fluorines in its formula.

Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.

Neptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is an orange volatile crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioactive. Neptunium hexafluoride is stable in dry air but reacts vigorously with water.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
References
- 1 2 LeBlond, Nicolas; Lutar, Karel; Žemva, Boris (2000-01-16). "The First Organoxenon(IV) Compound: Pentafluorophenyldifluoroxenonium(IV) Tetrafluoroborate". Angewandte Chemie International Edition. 39 (2): 391–393. doi:10.1002/(SICI)1521-3773(20000117)39:2<391::AID-ANIE391>3.0.CO;2-U. PMID 10649421.
- 1 2 3 Frohn, H. (2004-05-31). "C6F5XeF, a versatile starting material in xenon-carbon chemistry". Journal of Fluorine Chemistry. 125 (6): 981–988. doi:10.1016/j.jfluchem.2004.01.019.
- ↑ Gilles, T.; Gnann, R.; Naumann, D.; Tebbe, K. F. (1994-03-15). "2,6-Difluorphenylxenon(II)-tetrafluoroborat". Acta Crystallographica Section C. 50 (3): 411–413. doi:10.1107/s0108270193009898. ISSN 0108-2701.
- ↑ Naumann, D.; Butler, H.; Gnann, R.; Tyrra, W. (1993-03-01). "Arylxenon tetrafluoroborates: compounds of unexpected stability". Inorganic Chemistry. 32 (6): 861–863. doi:10.1021/ic00058a018. ISSN 0020-1669.