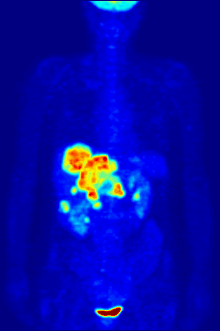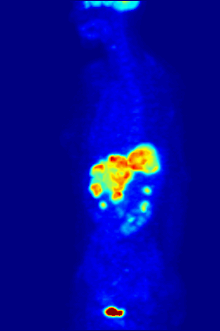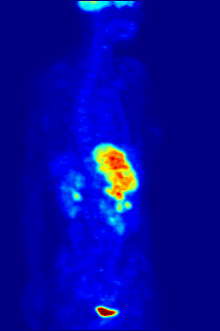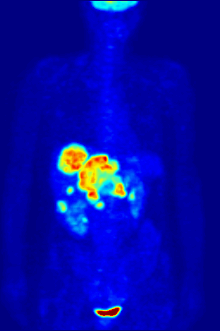
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.

A beta particle, also called beta ray or beta radiation, is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons respectively.

Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide. By virtue of its radioactive decay, it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling. In biological contexts, experiments that use radioisotope tracers are sometimes called radioisotope feeding experiments.

An autoradiograph is an image on an X-ray film or nuclear emulsion produced by the pattern of decay emissions from a distribution of a radioactive substance. Alternatively, the autoradiograph is also available as a digital image, due to the recent development of scintillation gas detectors or rare-earth phosphorimaging systems. The film or emulsion is apposed to the labeled tissue section to obtain the autoradiograph. The auto- prefix indicates that the radioactive substance is within the sample, as distinguished from the case of historadiography or microradiography, in which the sample is marked using an external source. Some autoradiographs can be examined microscopically for localization of silver grains in which the process is termed micro-autoradiography. For example, micro-autoradiography was used to examine whether atrazine was being metabolized by the hornwort plant or by epiphytic microorganisms in the biofilm layer surrounding the plant.
Liquid scintillation counting is the measurement of radioactive activity of a sample material which uses the technique of mixing the active material with a liquid scintillator, and counting the resultant photon emissions. The purpose is to allow more efficient counting due to the intimate contact of the activity with the scintillator. It is generally used for alpha particle or beta particle detection.
Phosphorus-32 (32P) is a radioactive isotope of phosphorus. The nucleus of phosphorus-32 contains 15 protons and 17 neutrons, one more neutron than the most common isotope of phosphorus, phosphorus-31. Phosphorus-32 only exists in small quantities on Earth as it has a short half-life of 14 days and so decays rapidly.

A radioimmunoassay (RIA) is an immunoassay that uses radiolabeled molecules in a stepwise formation of immune complexes. A RIA is a very sensitive in vitro assay technique used to measure concentrations of substances, usually measuring antigen concentrations by use of antibodies.
Isotopic labeling is a technique used to track the passage of an isotope through chemical reaction, metabolic pathway, or a biological cell. The reactant is 'labeled' by replacing one or more specific atoms with their isotopes. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.

Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products, and neutrons. Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years.

Radiochemistry is the chemistry of radioactive materials, where radioactive isotopes of elements are used to study the properties and chemical reactions of non-radioactive isotopes. Much of radiochemistry deals with the use of radioactivity to study ordinary chemical reactions. This is very different from radiation chemistry where the radiation levels are kept too low to influence the chemistry.

Radioluminescence is the phenomenon by which light is produced in a material by bombardment with ionizing radiation such as alpha particles, beta particles, or gamma rays. Radioluminescence is used as a low level light source for night illumination of instruments or signage. Radioluminescent paint is occasionally used for clock hands and instrument dials, enabling them to be read in the dark. Radioluminescence is also sometimes seen around high-power radiation sources, such as nuclear reactors and radioisotopes.
Various radionuclides emit beta particles, high-speed electrons or positrons, through radioactive decay of their atomic nucleus. These can be used in a range of different industrial, scientific, and medical applications. This article lists some common beta-emitting radionuclides of technological importance, and their properties.
An optoelectric nuclear battery is a type of nuclear battery in which nuclear energy is converted into light, which is then used to generate electrical energy. This is accomplished by letting the ionizing radiation emitted by the radioactive isotopes hit a luminescent material, which in turn emits photons that generate electricity upon striking a photovoltaic cell.

Molecular imaging is a field of medical imaging that focuses on imaging molecules of medical interest within living patients. This is in contrast to conventional methods for obtaining molecular information from preserved tissue samples, such as histology. Molecules of interest may be either ones produced naturally by the body, or synthetic molecules produced in a laboratory and injected into a patient by a doctor. The most common example of molecular imaging used clinically today is to inject a contrast agent into a patient's bloodstream and to use an imaging modality to track its movement in the body. Molecular imaging originated from the field of radiology from a need to better understand fundamental molecular processes inside organisms in a noninvasive manner.
Krypton-85 (85Kr) is a radioisotope of krypton.

Environmental radioactivity is produced by radioactive materials in the human environment. While some radioisotopes, such as strontium-90 (90Sr) and technetium-99 (99Tc), are only found on Earth as a result of human activity, and some, like potassium-40 (40K), are only present due to natural processes, a few isotopes, e.g. tritium (3H), result from both natural processes and human activities. The concentration and location of some natural isotopes, particularly uranium-238 (238U), can be affected by human activity.
Scintillation proximity assay (SPA) is an assay development and biochemical screening that permits the rapid and sensitive measurement of a broad range of biological processes in a homogeneous system. The type of beads that are involved in the SPA are microscopic in size and within the beads itself, there is a scintillant which emits light when it is stimulated. Stimulation occurs when radio-labelled molecules interact and bind to the surface of the bead. This interaction will trigger the bead to emit light, which can be detected using a photometer.

Fluorescence is used in the life sciences generally as a non-destructive way of tracking or analysing biological molecules. Some proteins or small molecules in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence. Alternatively, specific or general proteins, nucleic acids, lipids or small molecules can be "labelled" with an extrinsic fluorophore, a fluorescent dye which can be a small molecule, protein or quantum dot. Several techniques exist to exploit additional properties of fluorophores, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in properties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.
Radiofluorination is the process by which a radioactive isotope of fluorine is attached to a molecule and is preferably performed by nucleophilic substitution using nitro or halogens as leaving groups. Fluorine-18 is the most common isotope used for this procedure. This is due to its 97% positron emission and relatively long 109.8 min half-life. The half-life allows for a long enough time to be incorporated into the molecule and be used without causing exceedingly harmful effects. This process has many applications especially with the use of positron emission tomography (PET) as the aforementioned low positron energy is able to yield a high resolution in PET imaging.