Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involved in a wide range of physiological processes, including stress response, immune response, and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior.

Prednisone is a glucocorticoid medication mostly used to suppress the immune system and decrease inflammation in conditions such as asthma, COPD, and rheumatologic diseases. It is also used to treat high blood calcium due to cancer and adrenal insufficiency along with other steroids. It is taken by mouth.

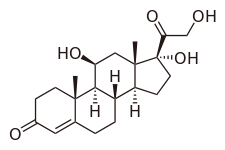
Hydrocortisone is the name for the hormone cortisol when supplied as a medication. Uses include conditions such as adrenocortical insufficiency, adrenogenital syndrome, high blood calcium, thyroiditis, rheumatoid arthritis, dermatitis, asthma, and COPD. It is the treatment of choice for adrenocortical insufficiency. It can be given by mouth, topically, or by injection. Stopping treatment after long-term use should be done slowly.

Glucocorticoids are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebrate animal cell. The name "glucocorticoid" is a portmanteau and is composed from its role in regulation of glucose metabolism, synthesis in the adrenal cortex, and its steroidal structure.

Prednisolone is a corticosteroid, a steroid hormone used to treat certain types of allergies, inflammatory conditions, autoimmune disorders, and cancers. Some of these conditions include adrenocortical insufficiency, high blood calcium, rheumatoid arthritis, dermatitis, eye inflammation, asthma, and multiple sclerosis. It can be taken by mouth, injected into a vein, used topically as a skin cream, or as eye drops. It differs from the similarly named prednisone in having a hydroxyl at the 11th carbon instead of a ketone.
Antipruritics, abirritants, or anti-itch drugs, are medications that inhibit the itching often associated with sunburns, allergic reactions, eczema, psoriasis, chickenpox, fungal infections, insect bites and stings like those from mosquitoes, fleas, and mites, and contact dermatitis and urticaria caused by plants such as poison ivy or stinging nettle. It can also be caused by chronic kidney disease and related conditions.

Clobetasol propionate is a corticosteroid used to treat skin conditions such as eczema, contact dermatitis, seborrheic dermatitis, and psoriasis. It is applied to the skin as a cream, ointment, or shampoo. Use should be short term and only if other weaker corticosteroids are not effective. Use is not recommended in rosacea or perioral dermatitis.

Triamcinolone is a glucocorticoid used to treat certain skin diseases, allergies, and rheumatic disorders among others. It is also used to prevent worsening of asthma and COPD. It can be taken in various ways including by mouth, injection into a muscle, and inhalation.

Betamethasone is a steroid medication. It is used for a number of diseases including rheumatic disorders such as rheumatoid arthritis and systemic lupus erythematosus, skin diseases such as dermatitis and psoriasis, allergic conditions such as asthma and angioedema, preterm labor to speed the development of the baby's lungs, Crohn's disease, cancers such as leukemia, and along with fludrocortisone for adrenocortical insufficiency, among others. It can be taken by mouth, injected into a muscle, or applied to the skin, typically in cream, lotion, or liquid forms.
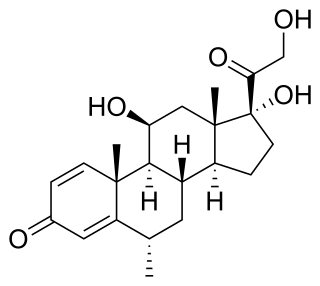
Methylprednisolone is a synthetic glucocorticoid, primarily prescribed for its anti-inflammatory and immunosuppressive effects. It is either used at low doses for chronic illnesses or used concomitantly at high doses during acute flares. Methylprednisolone and its derivatives can be administered orally or parenterally.

Desonide (INN) is a low-potency topical corticosteroid anti-inflammatory that has been available since the 1970s. It is primarily used to treat atopic dermatitis (eczema), seborrheic dermatitis, contact dermatitis and psoriasis in both adults and children. It has a fairly good safety profile and is available as a cream, ointment, lotion, and as a foam under the tradename Verdeso Foam. Other trade names for creams, lotions, and ointments include Tridesilon, DesOwen, Desonate. It is a group VI corticosteroid under US classification, the second least potent group.

Betamethasone dipropionate is a glucocorticoid steroid with anti-inflammatory and immunosuppressive abilities. It is applied as a topical cream, ointment, lotion or gel (Diprolene) to treat itching and other skin conditions such as eczema. Minor side effects include dry skin and mild, temporary stinging when applied. Betamethasone dipropionate is a "super high potency" corticosteroid used to treat inflammatory skin conditions such as dermatitis, eczema and psoriasis. It is a synthetic analog of the adrenal corticosteroids. Although its exact mechanism of action is not known, it is effective when applied topically to cortico-responsive inflammatory dermatoses. It is available as a generic medication.

Mometasone, also known as mometasone y 3 s, is a steroid medication used to treat certain skin conditions, hay fever, and asthma. Specifically it is used to prevent rather than treat asthma attacks. It can be applied to the skin, inhaled, or used in the nose. Mometasone furoate, not mometasone, is used in medical products.

Loteprednol is a topical corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax and Loterex.

Amcinonide is a topical glucocorticoid used to treat itching, redness and swelling associated with several dermatologic conditions such as atopic dermatitis and allergic contact dermatitis. Amcinonide can also be classified as a multi-functional small molecule corticosteroid, which has been approved by the FDA and is currently marketed as an ointment, lotion, or cream. It acts as both a transcription factor for responses to glucocorticoids and modulator for other transcription factors while also regulating phospholipase A2 activity.
In medicine, a finger tip unit (FTU) is defined as the amount of ointment, cream or other semi-solid dosage form expressed from a tube with a 5 mm diameter nozzle, applied from the distal skin-crease to the tip of the index finger of an adult. The "distal skin-crease" is the skin crease over the joint nearest the end of the finger. One FTU is enough to treat an area of skin twice the size of the flat of an adult's hand with the fingers together, i.e. a "handprint". Two FTUs are approximately equivalent to 1 g of topical steroid.
Topical steroids are the topical forms of corticosteroids. Topical steroids are the most commonly prescribed topical medications for the treatment of rash and eczema. Topical steroids have anti-inflammatory properties and are classified based on their skin vasoconstrictive abilities. There are numerous topical steroid products. All the preparations in each class have the same anti-inflammatory properties but essentially differ in base and price.

Selective glucocorticoid receptor modulators (SEGRMs) and selective glucocorticoid receptor agonists (SEGRAs) formerly known as dissociated glucocorticoid receptor agonists (DIGRAs) are a class of experimental drugs designed to share many of the desirable anti-inflammatory, immunosuppressive, or anticancer properties of classical glucocorticoid drugs but with fewer side effects such as skin atrophy. Although preclinical evidence on SEGRAMs’ anti-inflammatory effects are culminating, currently, the efficacy of these SEGRAMs on cancer are largely unknown.

Topical glucocorticoids are the topical forms of glucocorticoids. Topical glucocorticoids are used in the treatment of many skin conditions. They provide anti-inflammatory, antimitotic, and immune-system suppressing actions through various mechanisms.

An antiarthritic is any drug used to relieve or prevent arthritic symptoms, such as joint pain or joint stiffness. Depending on the antiarthritic drug class, it is used for managing pain, reducing inflammation or acting as an immunosuppressant. These drugs are typically given orally, topically or through administration by injection. The choice of antiarthritic medication is often determined by the nature of arthritis, the severity of symptoms as well as other factors, such as the tolerability of side effects.