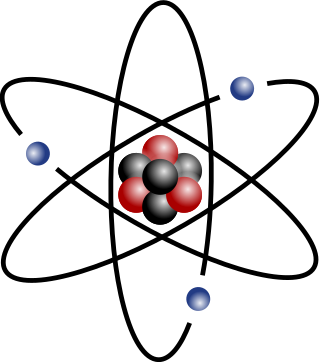
The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (np) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons.

An atom is a particle that consists of a nucleus of protons and neutrons surrounded by an electromagnetically-bound cloud of electrons. The atom is the basic particle of the chemical elements, and the chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. The number of neutrons defines the isotope of the element.

Atomic theory is the scientific theory that matter is composed of particles called atoms. The concept that matter is composed of discrete particles is an ancient idea, but gained scientific credence in the 18th and 19th centuries when scientists found it could explain the behaviors of gases and how chemical elements reacted with each other. By the end of the 19th century, atomic theory had gained widespread acceptance in the scientific community.
A chemical element is a chemical substance that cannot be broken down into other substances. The basic particle that constitutes a chemical element is the atom, and each chemical element is distinguished by the number of protons in the nuclei of its atoms, known as its atomic number. For example, oxygen has an atomic number of 8, meaning that each oxygen atom has 8 protons in its nucleus. This is in contrast to chemical compounds and mixtures, which contain atoms with more than one atomic number.

Deuterium is one of two stable isotopes of hydrogen. The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among every 6,420 atoms of hydrogen. Thus deuterium accounts for approximately 0.0156% by number of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another.

Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
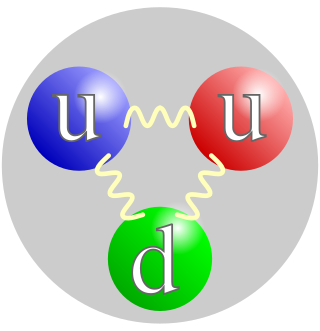
A proton is a stable subatomic particle, symbol
p
, H+, or 1H+ with a positive electric charge of +1 e (elementary charge). Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton-to-electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei).

William Prout FRS was an English chemist, physician, and natural theologian. He is remembered today mainly for what is called Prout's hypothesis.
Prout's hypothesis was an early 19th-century attempt to explain the existence of the various chemical elements through a hypothesis regarding the internal structure of the atom. In 1815 and 1816, the English chemist William Prout published two papers in which he observed that the atomic weights that had been measured for the elements known at that time appeared to be whole multiples of the atomic weight of hydrogen. He then hypothesized that the hydrogen atom was the only truly fundamental object, which he called protyle, and that the atoms of other elements were actually groupings of various numbers of hydrogen atoms.

The mass number (symbol A, from the German word: Atomgewicht, "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Since protons and neutrons are both baryons, the mass number A is identical with the baryon number B of the nucleus (and also of the whole atom or ion). The mass number is different for each isotope of a given chemical element, and the difference between the mass number and the atomic number Z gives the number of neutrons (N) in the nucleus: N = A − Z.

Francis William Aston FRS was a British chemist and physicist who won the 1922 Nobel Prize in Chemistry for his discovery, by means of his mass spectrograph, of isotopes in many non-radioactive elements and for his enunciation of the whole number rule. He was a fellow of the Royal Society and Fellow of Trinity College, Cambridge.
In chemistry, the amount of substance (symbol n) in a given sample of matter is defined as a ratio (n = N/NA) between the number of elementary entities (N) and the Avogadro constant (NA). The entities are usually molecules, atoms, or ions of a specified kind. The particular substance sampled may be specified using a subscript, e.g., the amount of sodium chloride (NaCl) would be denoted as nNaCl. The unit of amount of substance in the International System of Units is the mole (symbol: mol), a base unit. Since 2019, the value of the Avogadro constant NA is defined to be exactly 6.02214076×1023 mol−1. Sometimes, the amount of substance is referred to as the chemical amount or, informally, as the "number of moles" in a given sample of matter.

The Oddo–Harkins rule holds that an element with an even atomic number is more abundant than the elements with immediately adjacent atomic numbers. For example, carbon, with atomic number 6, is more abundant than boron (5) and nitrogen (7). This pattern was first reported by Giuseppe Oddo in 1914 and William Draper Harkins in 1917.
Although there are nine known isotopes of helium (2He), only helium-3 and helium-4 are stable. All radioisotopes are short-lived, the longest-lived being 6
He
with a half-life of 806.92(24) milliseconds. The least stable is 10
He
, with a half-life of 260(40) yoctoseconds, although it is possible that 2
He
may have an even shorter half-life.

Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always a positive number, as the nucleus must gain energy for the nucleons to move apart from each other. Nucleons are attracted to each other by the strong nuclear force. In theoretical nuclear physics, the nuclear binding energy is considered a negative number. In this context it represents the energy of the nucleus relative to the energy of the constituent nucleons when they are infinitely far apart. Both the experimental and theoretical views are equivalent, with slightly different emphasis on what the binding energy means.

The history of mass spectrometry has its roots in physical and chemical studies regarding the nature of matter. The study of gas discharges in the mid 19th century led to the discovery of anode and cathode rays, which turned out to be positive ions and electrons. Improved capabilities in the separation of these positive ions enabled the discovery of stable isotopes of the elements. The first such discovery was with the element neon, which was shown by mass spectrometry to have at least two stable isotopes: 20Ne and 22Ne. Mass spectrometers were used in the Manhattan Project for the separation of isotopes of uranium necessary to create the atomic bomb.

The mass recorded by a mass spectrometer can refer to different physical quantities depending on the characteristics of the instrument and the manner in which the mass spectrum is displayed.

Isotopes are distinct nuclear species of the same chemical element. They have the same atomic number and position in the periodic table, but differ in nucleon numbers due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties.
The atomic mass (ma or m) is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1 Da is defined as 1⁄12 of the mass of a free carbon-12 atom at rest in its ground state. The protons and neutrons of the nucleus account for nearly all of the total mass of atoms, with the electrons and nuclear binding energy making minor contributions. Thus, the numeric value of the atomic mass when expressed in daltons has nearly the same value as the mass number. Conversion between mass in kilograms and mass in daltons can be done using the atomic mass constant .
The discovery of the neutron and its properties was central to the extraordinary developments in atomic physics in the first half of the 20th century. Early in the century, Ernest Rutherford developed a crude model of the atom, based on the gold foil experiment of Hans Geiger and Ernest Marsden. In this model, atoms had their mass and positive electric charge concentrated in a very small nucleus. By 1920, isotopes of chemical elements had been discovered, the atomic masses had been determined to be (approximately) integer multiples of the mass of the hydrogen atom, and the atomic number had been identified as the charge on the nucleus. Throughout the 1920s, the nucleus was viewed as composed of combinations of protons and electrons, the two elementary particles known at the time, but that model presented several experimental and theoretical contradictions.