Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium and was thus named after the United States by analogy.
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.

Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory, by bombarding curium with alpha particles. It is an actinide element, the sixth transuranium element to be synthesized, and has the second-highest atomic mass of all elements that have been produced in amounts large enough to see with the naked eye. The element was named after the university and the U.S. state of California.
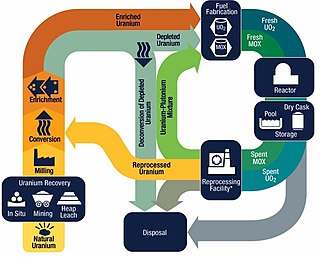
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.

Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactors. The reprocessed uranium, also known as the spent fuel material, can in principle also be re-used as fuel, but that is only economical when uranium supply is low and prices are high. Nuclear reprocessing may extend beyond fuel and include the reprocessing of other nuclear reactor material, such as Zircaloy cladding.
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the light-water reactors that predominate nuclear power generation.
A subcritical reactor is a nuclear fission reactor concept that produces fission without achieving criticality. Instead of sustaining a chain reaction, a subcritical reactor uses additional neutrons from an outside source. There are two general classes of such devices. One uses neutrons provided by a nuclear fusion machine, a concept known as a fusion–fission hybrid. The other uses neutrons created through spallation of heavy nuclei by charged particles such as protons accelerated by a particle accelerator, a concept known as an accelerator-driven system (ADS) or accelerator-driven sub-critical reactor.
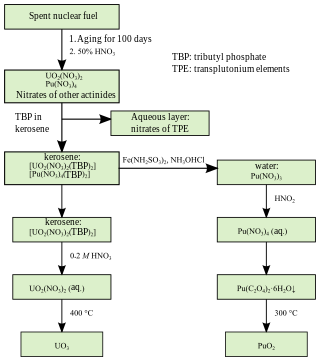
PUREX is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel. It is based on liquid–liquid extraction ion-exchange.
Fertile material is a material that, although not fissile itself, can be converted into a fissile material by neutron absorption.
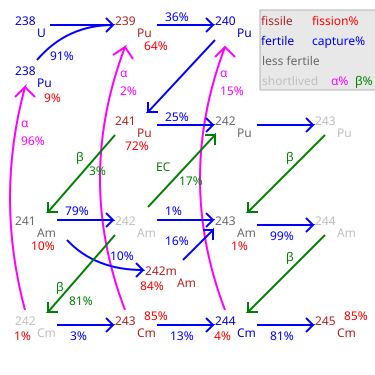
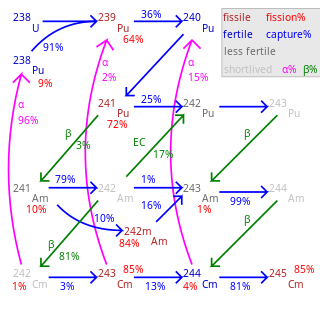
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being 238Pu in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are plutonium-244 with a half-life of 80.8 million years, plutonium-242 with a half-life of 373,300 years, and plutonium-239 with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states; all have half-lives of less than one second.
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by ejecting alpha particles. Americium has an atomic number of 95. Despite 243
Am being an order of magnitude longer lived than 241
Am, the former is harder to obtain than the latter as more of it is present in spent nuclear fuel.
Fluoride volatility is the tendency of highly fluorinated molecules to vaporize at comparatively low temperatures. Heptafluorides, hexafluorides and pentafluorides have much lower boiling points than the lower-valence fluorides. Most difluorides and trifluorides have high boiling points, while most tetrafluorides and monofluorides fall in between. The term "fluoride volatility" is jargon used particularly in the context of separation of radionuclides.

Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor. It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and, depending on its point along the nuclear fuel cycle, it will have different isotopic constituents than when it started.
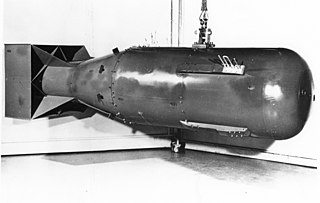
Weapons-grade nuclear material is any fissionable nuclear material that is pure enough to make a nuclear weapon or has properties that make it particularly suitable for nuclear weapons use. Plutonium and uranium in grades normally used in nuclear weapons are the most common examples.

Environmental radioactivity is not limited to actinides; non-actinides such as radon and radium are of note. While all actinides are radioactive, there are a lot of actinides or actinide-relating minerals in the Earth's crust such as uranium and thorium. These minerals are helpful in many ways, such as carbon-dating, most detectors, X-rays, and more.
Plutonium-241 is an isotope of plutonium formed when plutonium-240 captures a neutron. Like some other plutonium isotopes, 241Pu is fissile, with a neutron absorption cross section about one-third greater than that of 239Pu, and a similar probability of fissioning on neutron absorption, around 73%. In the non-fission case, neutron capture produces plutonium-242. In general, isotopes with an odd number of neutrons are both more likely to absorb a neutron, and more likely to undergo fission on neutron absorption, than isotopes with an even number of neutrons.
Plutonium-242 is one of the isotopes of plutonium, the second longest-lived, with a half-life of 375,000 years. The half-life of 242Pu is about 15 times that of 239Pu; so it is one-fifteenth as radioactive, and not one of the larger contributors to nuclear waste radioactivity. 242Pu's gamma ray emissions are also weaker than those of the other isotopes.
Long-lived fission products (LLFPs) are radioactive materials with a long half-life produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity, it is necessary to isolate them from humans and the biosphere and to confine them in nuclear waste repositories for geological periods of time.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.